Microscopic waste plastics are taken up by marine microbes to infiltrate and poison the entire food web in the oceans Dr. Mae-Wan Ho
Plastic waste pollution of our planet has reached crisis point, especially in the oceans (see [1] Plastic Plague in Our Oceans, SiS 65), where it poses unprecedented threat to marine life. Distressing photographs of marine mammals, birds, and amphibians mangled by plastic bags are all too familiar. Those are but the most visible signs of a much more extensive and insidious assault on life in and around the oceans. There is abundant literature on the danger of plastic waste to wild life at the top of the food chain, which has been further highlighted in numerous reviews (see [2-6] for example). Birds, sea turtles, and marine mammals, have all been found entangled in plastics, or with ingested pieces of plastics; as a result, they suffer impaired movement, ability to feed and reproduce; as well as lacerations, ulcerations and death.
In comparison, there have been few studies on microscopic pieces of waste plastics - mm to nm dimensions - that could affect increasingly greater numbers of species down the food chain.
Plastic wastes are not only physically harmful. They may be chemically harmful either because they are inherently toxic, or because they absorb other pollutants that are toxic. As pointed out in a recent review [6], microplastics can be ingested by suspension- filter- and deposit- feeders, detritivores and planktivores, all at the bottom of the food web. They may accumulate within the organisms, resulting in both physical and chemical damage. They can cause abrasions and blockages. And toxicity could arise from contaminants leaching from the microplastics such as monomers and plastic additives that are carcinogenic and/or endocrine disrupting. Moreover, microplastics can concentrate hydrophobic persistent organic pollutants (POPs) that have a greater affinity for the hydrophobic surface of plastics compared to seawater. On account of their large surface area to volume ratio, microplastics can become heavily contaminated, at up to 6 orders of magnitude greater than ambient seawater in the case of waterborne POPs [7, 8].
Several recent studies are beginning to address the effects of microplastics in the marine food chain.
A team of researchers at University of California Davis, and University of California, San Diego showed that fish exposed to a mixture of polyethylene with chemical pollutants adsorbed from the marine environment bioaccumulate these chemical pollutants and suffer liver toxicity and pathology [9]. Fish fed virgin polyethylene fragments also showed signs of stress, although less severe than fish fed marine polyethylene fragments.
Japanese medaka (Oryzias latipes), a widely accepted model fish species, were exposed chronically to low density polyethylene (LDPE) plastic (used for carrier bags). Polyethylene has a greater affinity for organic contaminants than other mass produced polymers, comprises the largest component of plastic production globally (29 %), and is one of the most common polymers recovered as aquatic debris.
The fish were exposed to three treatments: a negative control with no LDPE, a virgin-plastic treatment, and a marine plastic treatment (where LDPE was pre-exposed to water in an urban San Diego Bay). The medaka were given 10 % plastic (by weight) mixed into treatment diets and sprinkled at the top of each tank. This translates to 8 ng plastic/ml. of water, which is within the range of concentrations found in seawater (maximum reported in the North Pacific subtropical Gyre was 300 ng/ml). Chemical analyses targeted polycyclic aromatic hydrocarbons (PAHs), PCBs (polychlorinated biphenyls) and PBDEs (polybrominated biphenyls), all of which are known to accumulate on plastic debris in marine habitats. In addition, PBDEs are additives on several plastics and PAHs are a likely byproduct of plastic manufacturing.
The team probed for 12 PAHs, 27 PCBs and 13 PBDEs in virgin- and marine- LDPE pellets and detected and quantified 6 PAHs, 10 PCBs and 7PBDEs. The concentrations of total PAHs, PCBs and PBDEs on marine-LDPE were 4-, 15- and 1.4- fold respectively of those on virgin-LDPE pellets. Small amounts of all the contaminants were also in the control diet (contained in the cod liver oil).
After two months, there were greater concentrations of total PAHs, PCBs and PBDEs in fish from the marine-plastic treatment at 2.4-, 1.2-, and 1.8-fold respectively of those from negative control treatment; but the differences were not significant among treatments except for the concentrations of chrysene and PCB28. There were small differences in mortality: 4 % from negative controls and virgin plastic and 6 % from marine plastic.
Significantly, fish exposed to virgin and marine plastic showed signs of stress in their liver, including glycogen depletion, fatty vacuoles and single cell death (necrosis). Severe glycogen depletion was seen in 74% of fish from marine plastic treatment, 46 % from virgin plastic and 0% from controls. Fatty vacuolation was seen in 47% of fish from marine plastic treatment, 29 % of fish from virgin plastic and 21 % from controls. Single cell necrosis was 11 % of fish from marine plastic treatment compared to 0 % from virgin plastic treatment and controls. An eosinophilic focus of cellular alteration, a precursor to a tumour was see in one fish from the virgin-plastic treatment, while a tumour - a hepatocellular adenoma comprising 25 % of the liver - was seen in one fish from marine plastic treatment.
Summing up, the researchers wrote [9]:“Overall, we conclude that polyethylene ingestion is a vector for the bioaccumulation of PBTs [polybutylene terephthalates, a collective term for the PAHs, PCBs and PBDEs] in fish and that toxicity resulting from plastic ingestion is a consequence of both the sorbed contaminants and plastic material.”
Research led by Sara Linse at Lund University in Sweden showed that commercially manufactured polystyrene nanoparticles were transported through an aquatic food chain from algae to zooplankton to fish (see Figure 1). The polystyrene nanoparticles affected lipid metabolism and behaviour of the top consumer [10]. At least three independent metabolic parameters were found to differ between control and test fish: weight loss, triglycerides:cholesterol ratio in blood serum, and distribution of cholesterol between muscle and liver. Moreover, they demonstrated that the nanoparticles bind to apolipoprotein A-I (apoA-1) in fish serum in vitro, thereby preventing the fish from properly utilising their fat reserves. In addition to the metabolic effects, the feeding behaviour of the fish was profoundly affected. The time it took the fish to consume 95% of the food presented to them was more than double in the nanoparticle-exposed fish compared to controls.
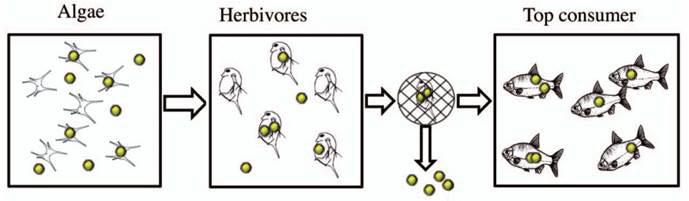
Figure 1 Experiment simulating the accumulation of polystyrene nanoparticles in the ocean in fish via the food chain
Polystyrene nanoparticles 24 nm in diameter were added at a concentration of 0.01% (w/v) to a culture of the green (Scenedesmus sp) for 24 h, filtered, and fed to herbivorous zooplankton (algae from 250 ml culture given to 30 adult Daphnia). After another 24 h, the Daphnia were washed on a net to remove free nanoparticles before being fed to the top consumers of the food chain, the Crucian carp, 4 individuals per replicate tank. The food chain was restarted every third day and the fish remained the same throughout the study. The control food chain worked in the same way except that no nanoparticles were added. Each food chain started with 16 fish divided into four tanks. The number of fish in each tank decreased over time as they were sacrificed for sampling.
The feeding time – the time it took for the fish to eat 95 % of the Daphnia added to the tank – was measured at day 18, 21, 24, 27 and 30. The average feeding time over the five time points were more than twice as long for fish exposed compared to controls (16.6 + 2.7 versus 6.0 + 0.7 minutes, p < 0.035). The exposed fish were moving more slowly and to a much lesser extent than control fish and they did not actively hunt for the zooplankton during the feeding. Remarkably, the test fish let Daphnia swim in and out of their mouth without trying to eat them. This indicates a strong behavioural disturbance in fish that have eaten food containing the nanoparticles.
To identify proteins that bind to polystyrene particles in fish serum, the researchers incubated polystyrene nanoparticles with serum collected from the Crucian carp and several fish species: pike, tench, rudd, bleak, and Atlantic salmon. For all fish species investigated, one of the main proteins bound to the nanoparticles migrates as expected for a protein with molecular weight around 25 kDA. The protein band from Atlantic salmon (Salmo solar), whose genome is the only one that has been sequenced, was cut out and subjected to trypsin proteolysis, followed by mass spectrometry, and identified as apoA-I, the carrier protein for high density lipoprotein (HLP), an important component for fat metabolism. Unlike mammals which use glycogen as the main energy reserve, fat is the major energy reserve for fish.
It is possible that the polystyrene nanoparticles bind to both apoA-I and the HDL particles in serum after being ingested and passed through the intestinal wall into the blood stream, and from there to other tissues, where it may have further influences on lipid metabolism. The researchers therefore investigated changes in the concentrations of triglycerides and phospholipids in blood serum, liver and muscles throughout the experiment. They found two significant differences between the test and control fish. The triglyceride:cholesterol ratios in blood serum were similar after 14 days. After 22 days, the ratio for the control fish was very low whereas only a small decrease was seen in the test fish. After 29 days, the ratios had increased for both the control and test group. In addition, the distribution of cholesterol among muscle and liver changed during the experiment. After 14 days, the distribution of cholesterol was the same in control and test fish. However, after 22 days, the cholesterol concentrations in the control group were elevated in muscle and liver by comparison. After 29 days, the distribution of cholesterol was again similar. These results indicated that control fish were able to alter their fat metabolism to cope with the spare dietary regime, whereas the test fish were not.
Throughout the experiment, the weight of the fish was measured. As the fish is fed with a limited amount of zooplankton, a weight loss is expected because the fish is forced to use its energy reserves. A significant weight loss was observed in both test and control fish from the first feeding to the 15th day. Between day 15 and 21, the weight loss slowed down for both groups. After this, the control group continued to lose weight whereas the test fish actually gained weight at the end of the experiment. A possible explanation is that the test fish were unable to draw upon their energy fat reserve due to the accumulation of nanoparticles.
Taken altogether, the results suggest that there is a disturbance of lipid metabolism as a consequence of nanoparticle intake, which make the fish less adaptable to the starvation diet (and may also account for their inactivity during feeding due to lack of energy).
The behaviour of the fish were further investigated by video recording as described in a subsequent publication of a follow up study carried out in collaboration with scientists at University of Copenhagen, Denmark [11] with the same experimental design.
Commercially available suphonated polystyrene nanoparticles 24 and 27 nm in diameter were used at a concentration of 0.01 % (w/v), or 9.3 x 1012 particles/ml. The number of nanoparticles reaching each fish was calculated to be approximately 1 x 1013.
The behaviour of the fish was monitored by filming during feeding on day 0, 24, and 61. Each aquarium (with four fish) was filmed for 30 min. Blood and organs were also taken for analysis at the end of the experiment.
The study confirmed that feeding time was almost twice as long in test fish as in controls. During feeding, the control fish showed significantly higher activity than the test fish as they actively searched for food. The test fish moved much more slowly and did not hunt as actively for food. The distance between fish in each aquarium was smaller for test fish than controls, suggesting that the nano-particle fed fish behaved more as a group and exhibited stronger shoaling behaviour. The nano-particles fed fish occupied less of the available space in the aquarium than control fish during feeding, as consistent with their relative inactivity.
Fish organs blood, brain, gills, liver and muscle samples were analysed by NMR spectroscopy to identify effects of the nanoparticles diet on metabolite concentrations. The nanoparticles-fed fish showed increase in ethanol in the liver and decrease in muscle, and increases in inosine/adenosine and lysine in muscle, and also increases in leucine, phenylalanine and tyrosine in the liver. These results suggest a collective effect of many metabolite changes as the result of a general disturbance of cellular function.
The brain and the muscle differed between the groups, both in texture and colour. The brains in the nanoparticle-fed fish were much more fluffy, whiter, and appeared swollen, and significantly heavier than the brains of control fish, as more water was present.
The fish were given a restricted diet to test the effects of nanoparticles when they needed to use their fat reserves. The more pronounced decrease in activity of the nanoparticle-fed fish suggests that their energy reserves were smaller or that the access to the reserves was impaired.
Taken together, the results show that polystyrene nanoparticles induce considerable changes in metabolism and hunting behaviour. A reduced feeding activity may result in reduced growth and ability to avoid predators and thereby reduce their fitness in the natural ecosystem.
There is no longer any doubt that waste plastics in the ocean are a menace to all marine life. Especially insidious are the invisible micro- and nano-plastics that can be taken up by microorganisms at the bottom of the food web and bio-accumulate in top predators with profound effects on their feeding behaviour, metabolism and morphology, as evident from the first studies that have been carried out. The effects on other species lower down the food web have yet to be determined, but the effects on human health can already be predicted from existing data on the carcinogenic and endocrine disrupting effects of POPs and numerous chemical associated with waste plastics. There is an urgent need to drastically reduce plastic pollution by classifying plastic wastes hazardous (see [1]).
Article first published 02/02/15
Comments are now closed for this article
There are 4 comments on this article.
Désirée Röver Comment left 3rd February 2015 02:02:20
Greetings, Mae-Wan! Thank you for this sad article.
This plastic toxicity is bad enough, but what about the radioactive contamination that daily is being spewed into the waters of the Pacific Ocean?
The multitude of photographs by Dana Durnford shows that in some regions of the West coast of Canada marine life already is almost totally extinct (www.thenuclearproctologist.org).
And next to chemical toxicity, doesn't radioactivity also accumulate in the plastic materials mentioned in this article?
Adrian S Hepworth Comment left 3rd February 2015 03:03:07
You may be interested in this short film which my daughter helped make for TED Ed as the animator.
http://ed.ted.com/lessons/the-nurdles-quest-for-ocean-domination-kim-preshoff It tries to make this polution problem understandable by all.
Rory Short Comment left 3rd February 2015 05:05:50
Nature through 3.8 billion years of evolution produced a biosphere where everything worked together effectively for the survival of the whole. One of Nature's products, humankind, has however, in its arrogance, seen itself as being beyond Nature. Consequently humankind does not even try to consult the wisdom of Nature before embarking on activities which will effect the biosphere in new ways. Man made chemicals are one such product and Nature was and is not consulted when they are released. Such consultation should be mandatory before a product can be released into the biosphere
victoria lewis Comment left 30th March 2016 11:11:50
where does polyester fit into this research? I have read stuff that suggests it is a very significant contributor to ocean pollution.