The ‘Deadly Dangers of Saturated Fat’ & the ‘Superlative Safety of Statins’ Part 3
The efficacy of statins was hyped and serious side effects under-reported in sponsored studies both flawed and totally lacking transparency Dr Paul J Rosch
As documented in Parts 1 & 2, no studies had ever shown that dietary fat increased cholesterol or that lowering cholesterol prevented coronary atherosclerosis. This changed with the advent of statins, especially Lipitor (atorvastin), which reported a 36 % reduction in heart attacks by using relative risk statistics rather than actual risk of l %. Crestor was more potent than Lipitor but it was also associated with more adverse side effects. In March 2004, only eight months after its release, the Public Citizen consumer advocacy group asked the FDA to ban Crestor. Three cases of kidney failure associated with severe rhabdomyolysis (destruction of striated muscle) and one death had already occurred in the U.S. and seven cases of rhabdomyolysis and nine of kidney failure had been reported in Canada and the UK. That was ominous, as is well established the vast majority of adverse drug reactions are never reported. Baycol, an early statin, was approved by the FDA in 1997 and by the time it was banned in 2001, 1,899 cases of rhabdomyolysis and numerous deaths from kidney failure had been documented, many of which might have been prevented since they occurred long after unequivocal evidence that the drug should have been withdrawn. A 2005 study revealed that kidney failure and muscle weakness were two to eight times more frequent among Crestor users than those taking Lipitor and Zocor (simvastatin) [1].
Nevertheless, there allegedly were no unusual side effects or elevation of liver enzymes from the 40-mg. maximal dose of Crestor taken daily for two years in the ASTEROID trial. This was the first study to show a regression of plaque volume based on intracoronary ultrasound (IVUS) measurements after treatment, as shown below in Figure 1.
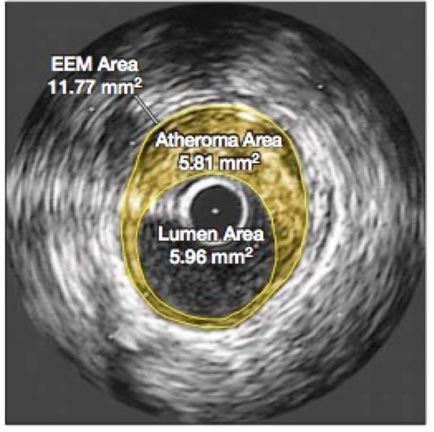
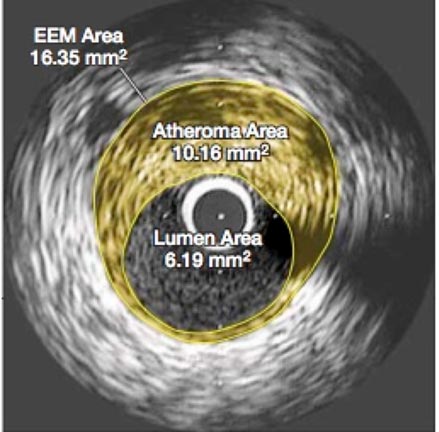
Figure 1 Effect of very high-intensity statin therapy on regression of coronary atherosclerosis in the ASTEROID trial; left before treatment, right after treatment [2]
It was the cross-sectional areas of atheroma that were compared before and after treatment, as it was assumed that these measurements were directly proportional to their volumes and that that the area of the lumen would show a corresponding increase. However, what was not discussed in either the press releases or the article was that the lumen area actually decreased by 4 %, and as can be seen, the images also showed that the arterial wall had thickened. This might not be beneficial because a smaller lumen and a stiffer arterial wall would both tend to increase blood pressure, an effect also not addressed in the published report. The Comments section conclusion said [2], “This very intensive statin regimen was well tolerated”. But the total dropout rate appears to have been 25 %; no details were provided to explain that. Also, the trial may have been too short for the Crestor side effects reported in other studies to have surfaced, raising questions about long-term safety with this large daily dose that would have to be taken perpetually. In addition, there were no controls and only 349 patients.
Crestor really made headlines with the subsequent JUPITER trial in men over 50 and women over 60 with no history of heart disease but who had an elevated C-Reactive Protein (CRP) and a normal LDL [3]. These 17 802 healthy people received either 20 mg of Crestor daily or a placebo and were followed to document the occurrence of the following end points: fatal and nonfatal myocardial infarction, fatal and nonfatal stroke, arterial revascularization, hospitalization for unstable angina or death due to a confirmed cardiovascular cause. It was scheduled to run until 520 end points had been reached. However, the way end point statistics were collected, one person might be recorded as having several, even though they had only one incident or hospitalization. There was so much overlap that it is difficult to know exactly what was being reported. It was anticipated that the study might last four or five years, but it was stopped after 23 months at which time only 393 end points had been reported. Although an “unequivocal reduction in cardiovascular mortality” was publicly announced as the major reason, the data did not support that. The only justification for termination was that the placebo group had experienced 109 more of these confusing end points and it was felt that continuing the study could subject them to increased harm.
This was exaggerated in reports claiming that Crestor reduced by almost 50% the risk for a major first cardiovascular event; but this was relative risk (RR) As can be seen below, the actual risk reduction (AR) was less than 1%.
Rate of primary endpoint: Crestor 1.6%; placebo 2.8% → AR, 1.2%.
Rate of fatal or nonfatal MI: Crestor 0.35%; placebo 0.76% → AR 0.41%.
Rate of fatal or nonfatal stroke: Crestor 0.37%; placebo 0.72% → AR 0.35%
It would appear from the above that the Crestor group indeed had slightly less than half as many heart attacks, but they also had 150 % more fatal heart attacks. The data was presented in a manner that obscured this, as follows:
Any myocardial infarction: Crestor 31, Placebo 68
Nonfatal myocardial infarction: Crestor 22, Placebo 62
To find the number of fatal heart attacks, subtract "Nonfatal myocardial infarction" from "Any myocardial infarction". This reveals 9 deaths in those receiving Crestor (29 %), compared to 6 in the placebo group (9%). Stroke was similarly presented to show a 50 % reduction from Crestor:
Any stroke: Crestor 33, Placebo 64
Nonfatal stroke: Crestor 30, Placebo 58
This means there were 3 fatal strokes in the Crestor cohort and 6 in those taking a placebo, so that total cardiovascular deaths (12) were identical in both groups.
There were also concerns about conflicts of interest and the role of the sponsor. The lead author is a co-holder of the patent for the hsCRP test used, which became the standard method of measurement at $50.00/test. Nine of the 14 authors had significant financial ties to AstraZeneca, whose investigators also collected, controlled and managed the raw data and monitored the collection sites. It is well established from other drug company sponsored studies that bias can creep in, such as the preponderance in the placebo group of patients with a family history of heart disease or metabolic syndrome, both of which significantly increase risk. Crestor is a potent statin with numerous side effects, but in JUPITER, there were just as many side effects in the placebo group. The most common adverse side effect is musculoskeletal pain, which occurs in 25 % [4]. But only 19 out of the 18 000 subjects taking part in the trial reported this symptom, 10 in the Crestor group and 9 in the placebo group. At the time the study was terminated, one out of four were not taking their medication. No reason was given for this nor do we know why or how many of the deaths came from this group. The fact is that there was a difference of less than 50 deaths between the two groups during the study, which given the large number of participants, means that neither group was at a significantly increased risk of dying. And the gap seemed to be closing. Many feel this may explain why JUPITER was terminated prematurely, as a longer period of observation might have shown no difference or even more deaths in the Crestor group, as illustrated below in Figure 2.
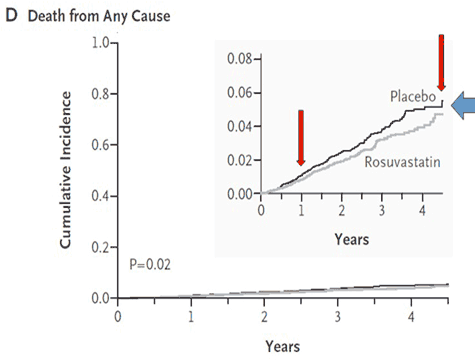
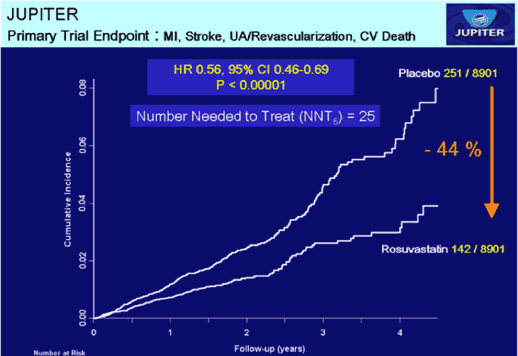
Figure 2 In the all cause mortality graph to the left, the bottom two curves for Crestor and placebo are almost identical, which happens when very small numbers are involved. On the two divergent curves at the top, the authors had to use a different scale to make it appear that there was a major difference. The primary trial endpoint graph to the right was manipulated by utilizing the same tactic [3]
As previously noted, the end point tabulation was confusing as a patient could have more than one and some, such as death, were more important than being hospitalized several times for angina. The patient population was also unusual, since it would be difficult to assemble almost 18 000 people over the age of 50 or 60 with both an LDL under 130 and an elevated CRP without any history of heart disease or inflammatory disorder. It is thus not surprising that 1 315 physicians in 26 different countries were required, which averages out to a paltry 13 subjects per center, raising additional concerns.
There were numerous other criticisms, such as bias because of significant conflicts of interest [5], and [6] “the positive predictive value of hsCRP was only 1.35 % (241 events in 17 802 patients) in JUPITER. This makes it a rather weak predictor of cardiovascular risk.” In addition [7], “The present data suggest that the all-cause mortality reduction of 20 % reported in JUPITER is likely to be an extreme and exaggerated finding, as often occurs when trials are stopped early.” The justification for premature cessation is dubious, as cardiovascular deaths were the same in each group, and why would a trial be stopped because of possible increased danger to those taking a placebo? However, as was pointed out in an accompanying editorial, early termination [8] “provides inflated estimates of benefits, understates harms, allows findings to be published (and hence used to advantage in marketing) earlier, and reduces the cost of the trial - all significant benefits to an industry sponsor and a financially invested research team.”
With respect to minimizing adverse effects, JUPITER was the first placebo-controlled clinical trial to document that statins (Crestor) increased risk of diabetes 25 % [9]. The lead author dismissed this by claiming that the cardiovascular benefits far outweighed any possible harm, even though adult diabetics are 2 to 4 times more likely to develop heart disease or a stroke [10]. Had the trial continued, the incidence of diabetes might have increased significantly based on subsequent surveys. One involving 153 840 postmenopausal women followed for 12 years reported that those taking statins had a 48 % increased risk of diabetes compared to controls not taking statins [11]. Another, published earlier in 2015, found almost a 50 % increase in Type 2 diabetes in white men after taking statins for six years [12]. And in April 2015, an analysis of data from 26 000 beneficiaries of Tricare, the military health system, revealed that those taking statins for an elevated cholesterol were 87 % more likely to develop diabetes after 8.5 years [13]. This involved only people who at baseline were free of heart disease, diabetes, and other severe chronic disorders. Those taking statins were also 250 % more likely to develop diabetes with complications than non-statin-using diabetics. Nevertheless, current recommendations are that all diabetics 40 and older with no other cardiovascular risk factors should take a moderate-intensity statin forever, and those with other risk factors should receive a high-intensity dose to reduce LDL by at least 50 %.
JUPITER was largely responsible for changing the guidelines for cholesterol lowering drugs, as in addition to adult diabetics, most healthy people over the age of 50 were now eligible for statins. More importantly, CRP was established as a significant risk factor for coronary disease. Lowering an elevated LDL as much as possible was no longer the primary goal, and cholesterol was not even mentioned because it was evident that the cardioprotective effects of statins were not dependent on their levels. Other studies claimed that statins could prevent or be used to treat numerous disorders, including cancer, Alzheimer's, Parkinson's, multiple sclerosis, arthritis, cirrhosis and arthritis [14]. As those benefits were also entirely unrelated to lipid lowering, they were attributed to anti-clotting, anti-inflammatory and other pleiotropic effects.
A 2007 review of 192 published statin trials revealed that those funded by industry were 20 times more likely to produce favorable results than others without any conflict of interest [15]. As explained in Bad Pharma: how drug companies mislead doctors and harm patients this can be achieved in numerous ways, including stopping the study prematurely so that longer-term effects are not examined [16]. JUPITER was terminated prematurely on the recommendation of its Independent Data Monitoring Board chaired by Rory Collins, Professor of Medicine and Epidemiology at the University of Oxford and head of Cholesterol Treatment Trialists (CTT) Collaboration. Independent implies not being influenced or affected by others but there is little doubt that Collins was and is very biased about statin safety. With respect to the National Institute for Health and Clinical Excellence (NICE) recommendations that everyone 60 and older should be eligible for statins, his comment was [17], “some may not like the idea of mass medication but they can be reassured at least that statins have been found to have “virtually no side effects.” Because of this purported superb safety profile, a polypill containing a statin and three antihypertensive drugs is being proposed for everyone 55 years and older and adding statins to drinking water has also been suggested.
As described in detail in a recent post [18 and references therein] Statins for the Healthy are Harmful, SiS 66), in 2014, Collins demanded that two articles in the British Journal of Medicine that were critical of statins safety be retracted. This was rejected and may have backfired; he revealed in an e-mail that his CTT team “had assessed the effects of statins on heart disease and cancer but not other side effects such as muscle pain.” In another letter questioning conflicts of interest for the authors of the two papers, he claimed that CTT had no commercial funding and was supported by the Medical Research Council and British Heart Foundation. Two weeks later, “in a spirit of reciprocity” he wrote another letter stating that all grants CTT had received over the last two decades totaled £268 million (416 million dollars). An investigation of this revealed that Merck had contributed £217.5 million (338 million dollars), and it may be no coincidence that Merck's Zocor (simvastatin) was the most popular statin in the UK until its patent expired. With respect to the two official sponsors, The British Heart Foundation, which receives financial support from two dozen pharmaceutical and food companies, made 5 donations, the largest being £2.7 million. The Medical Research Council, which also receives funding from drug companies, made only 2 donations, the largest being £ 9.6 million. Thus, 80 % of CTT funding came from Merck, and a sizeable amount of the remainder was also likely from drug companies.
The CTT was established solely for the purpose of retrieving and reviewing all the data on statins. It has no other function. Collins had done such an admirable job in predicting that prescribing statins more widely could save 2 000 lives a year in Britain and prevent 10 000 heart attacks and strokes, that he was knighted in 2010. But with respect to safety, he had only examined the possible risk of dying, suffering a heart attack or developing cancer. Last February, due to mounting criticism, he announced that he would now review all the previous studies to investigate musculoskeletal pain, type 2 diabetes, memory loss, GI complaints, and other known side effects. However, his database includes only company-sponsored trials and he refuses to let others see the raw data. As Professor Colin Baigent, a spokesperson for CTT, who received such a request explained [19], “It is important to recognise that data from participating trials are not owned by the Collaboration [CTT], but remain the property of the trial sponsors, so we are not able to provide unlimited access to the combined database.” Baigent has acknowledged receiving financial support from Astra Zeneca, Merck, Sharp & Dohme, GSK and Johnson & Johnson. The bottom line is that CTT, which is funded primarily by drug companies, has repeatedly claimed that statins have no adverse side effects, will be reviewing only company-sponsored trials for complications that should have been looked into previously, and refuses to let others see the data that support their conclusions.
Reducing CRP, a marker of inflammation, best explained the benefits of Crestor in JUPITER, and since some claimed that CRP also caused atherosclerosis, it was proposed that coronary disease was due to inflammation rather than lipid deposits [20]. There was nothing new about this, since the renowned pathologist Rudolph Virchow, who was the first to demonstrate that presence of cholesterol in atheroma in 1856, described
atherosclerosis as “endarteritis deformans” [21]. The suffix “it is” emphasized that
it resulted from an inflammatory process that injured the inner lining of the
arteries, and that the cholesterol deposits started to appear subsequently.
Virchow was very specific about this when he wrote [21, 22]:
We cannot help regarding the process as one which has arisen out of irritation of the parts stimulating them to new, formative actions; so far therefore it comes under our ideas of inflammation, or at least of those processes which are extremely nearly allied to inflammation.….We can distinguish a stage of irritation preceding the fatty metamorphosis, comparable to the stage of swelling, cloudiness, and enlargement which we see in other inflamed parts. I have therefore felt no hesitation in siding with the old view in this matter, and in admitting an inflammation of the inner arterial coat to be the starting point of the so-called atheromatous degeneration.
Thus, atherosclerotic plaque was a response to inflammation and the cholesterol deposits came later. Notice that he described this process as “coming under our ideas of” and “nearly allied to inflammation”, but avoided calling it inflammation. That was because
2 000 years earlier, Celsus had defined inflammation as “calor, dolor, rubor, and tumor" (heat, pain, redness and swelling), to which Virchow had added “functio laesa” (disturbance of function). These were all signs and symptoms that could be seen or felt. In contrast atherosclerosis was silent and could only be verified by microscopic examination.
So how can we define this type of inflammation other than an elevated CRP? If statins work by reducing inflammation, then why was the powerful anti-inflammatory drug Vioxx recalled because of increased heart attacks, strokes and sudden death? As the Nobel Laureate Richard Feynman emphasized [23], “I learned a long time ago the difference between knowing something and the name of something.”
Coronary heart disease is a multifactorial disorder that can have many causes, including stress, homocysteine, infections, and free radical damage. Other contributing factors that influence susceptibility range from family history, age, gender, diabetes, hypertension and smoking, to sex hormones, obesity, physical activity, and alcohol consumption. Many of these are interrelated and some individuals have more than one. It would be naive to believe that CRP levels reflect an accurate assessment of these diverse risk factors, or that lowering CRP will safely and effectively reduce coronary mortality in healthy people. Association never proves causation. Treating an elevated CRP would simply repeat the same mistake g made by attempting to lower LDL and/or raise HDL based on the fallacious belief that these are bad or good.
As Albert Einstein is reputed to have noted, “We live in a world where it is easier to break an atom than a preconceived idea.”
Cholesterylester transfer protein (CETP) drugs that substantially increase HDL have failed to reduce the risk of atherosclerosis in two trials that either caused an increase in deaths (torcetrapib), or showed no clinical improvement despite significant HDL increases (dalcetrapib), but others are in the pipeline [24]. PCSK9 monoclonal antibody inhibition is being developed for patients on statins who have not obtained optimal LDL levels. These PCSK9 inhibitors are given by injection every 2 to 4 weeks; with an anticipated initial cost of $6 000 to $7 000/year, and long-term safety and efficacy has not been established [25]. The FDA approved apheresis for the treatment of high cholesterol in 2013 [26]; this is a procedure that removes LDL, VLDL and Lp(a) bad cholesterol from the blood by direct adsorption. Like kidney dialysis, the 2-3 hour treatment must be repeated weekly or every 2 weeks at a cost of $2 500/treatment (although there may be insurance coverage if the LDL is over 300 mg/dL or over 200 mg/dL in patients with coronary disease not responsive to statins).
Einstein had a large sign on the wall of his Princeton office with the following warning: “Not everything that can be counted, counts, and not everything that counts can be counted.” The first part of this statement applies to CRP and LDL, which are easy to measure, but may have little significance. The second part pertains to our inability to define, much less measure something we call “inflammation”, but which may include different types of pro-inflammatory processes. I am not optimistic about future progress, as there is so much money to be made by perpetuating the cholesterol hypothesis, instead of doing proper research and unbiased investigations. As Upton Sinclair noted [27], “It is difficult to get a man to understand something, when his salary depends on his not understanding it.”
Article first published 01/07/15
Comments are now closed for this article
There are 3 comments on this article.
Rory Short Comment left 2nd July 2015 03:03:41
One thing that has become clear to me from reading these three articles is that money and scientific integrity are not compatible.
Liz Comment left 2nd July 2015 03:03:35
Adding statins to drinking water could well precipitate a disaster far, far worse than Thalidomide. A research letter published in the April 8 issue of the New England Journal of Medicine described that 20 of 52 babies exposed to statins in the womb were born with serious neurological malformations. It's reported here http://weeksmd.com/2008/01/cholesterol-drugs-tied-to-birth-defects/
Molly Comment left 10th April 2016 11:11:08
I had several strokes, and was prescribed Crestor. Have taken it faithfully for several years but in the last couple of years I have noted pain and weakness in my muscles. Advised Dr. I do not wish to be on pain medication there fore wish to know the side affects of stopping this medicine? Molly T. Dalstrom