New age of water
Water has come of age. It is cool on everyone's lips. Decades of research on water is giving us remarkable insights into its dynamic collective structure, and changing our big picture of life and living process.
Organisms are seventy to eighty percent water. Is this water necessary to life? What vital functions does it serve?
Entire biochemistry and cell biology textbooks are still being written without ever mentioning the role of water. It is simply treated as the inert medium in which all the specific biochemical reactions are being played out.
Instead, recent findings are raising the possibility that it is water that’s stage-managing the biochemical drama of life. Water is life, it is the key to every living activity. Some people will even say it is the seat of consciousness.
I-SIS brings you the latest revelations on water in this extended series that starts from the basics.
Dr. Mae-Wan Ho reports on how a body of water appears to change as a whole and wonders if oceans do it too
Decades of bombarding water with X-rays and neutron beams have convinced most scientists that there is no long-range order in water. And although extended networks of hydrogen-bonded molecules are present, these networks are simply the result of local interactions between molecules at close range.
However, other measurement techniques are beginning to yield results suggesting that bodies of water behave as coherent wholes, in other words, their collective structure extends globally to all the molecules. One such technique, NMR (Nuclear Magnetic Resonance), measures chemical shifts of the nuclei of certain atoms by their response to radio waves when placed in a strong magnetic field (see Box 1).
The atomic nucleus in a molecule is influenced by other particles that are charged and in motion. NMR spectroscopy can therefore distinguish one nucleus from another and reveal the chemical surroundings of a nucleus. The NMR chemical shift is known to be very sensitive to intra- and intermolecular factors, and hence capable of give information concerning collective phases of molecules.
Chemists S.R. Dillon and R.C. Dougherty in Florida State University, Tallahassee, in the United States, looked at the changes in NMR chemical shifts of salts dissolved in water, and came up with some interesting results, which led them to conclude that, "the entire solution is a single electronic whole".
| Box 1 NMR and NMR chemical shift Nuclear Magnetic Resonance (NMR) is the absorption of electromagnetic radiation of a specific (resonant) frequency by an atomic nucleus placed in a strong static magnetic field, used especially in spectroscopic studies of molecular structure, and in medicine to measure rates of metabolism. Only atomic nuclei with an odd number of neutrons, such as 1H and 13C can be detected with NMR. These have spins of +1/2 and -1/2 in the presence of a static magnetic field. The nuclei can take up one of the two orientations, a low-energy, +1/2, orientation aligned with the magnetic field, or a high-energy orientation, -1/2, opposed to the magnetic field. When the sample is now exposed to electromagnetic radiation of a certain frequency corresponding to the difference in energy between these two orientations, a few of the low-energy +1/2 nuclei absorb enough energy to rise to the high-energy -1/2 state. This absorption is called resonance, and is detected by an NMR spectrophotometer as a peak. The atomic nucleus in a molecule is influenced by other particles that are charged and in motion. The NMR chemical shift, d, is expressed in parts per million (ppm) with respect to a standard compound which is defined to be at 0 ppm, as follows:
NMR spectroscopy can distinguish one nucleus from another and reveal the chemical surroundings of a nucleus. The NMR chemical shift is known to be very sensitive to intra- and intermolecular factors, and hence capable of give information concerning collective phases of molecules. |
The NMR chemical shift of a salt goes up as its concentration increases. However, when the chemical shift is plotted against the concentration, there is typically a sharp change in the slope of the curve at certain critical concentrations. For a solution of KF (potassium fluoride), the chemical shifts for both 19F and 39K (the number in superscript identify the particular isotope of the element) increases linearly from 1.9 to 2.4 mol per litre, then changed abruptly to a different slope thereafter (see Fig. 1).
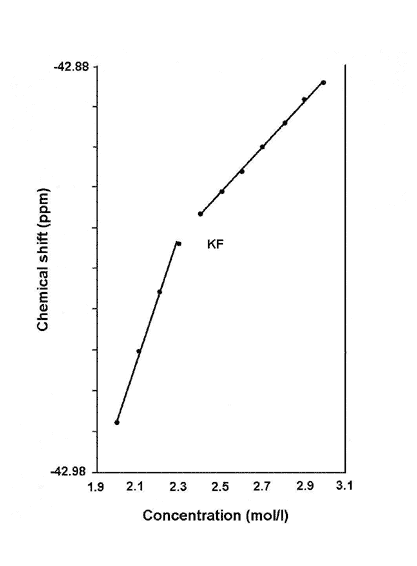
Figure 1. Change in chemical shift with concentration.
Similarly, the chemical shift of 39K in KCL (potassium chloride) solution showed a break in slope around 1.7 mol per litre, while the chemical shift of 7Li in LiOH (lithium hydroxide) solution changed in slope at 3.0 mol per litre.
These changes in the slope of chemical shifts with concentration are correlated with corresponding changes in the specific heat of the electrolyte (salt) solutions. The specific heat of pure water changes with temperature, starting at high levels below 280K and drops to a minimum at around 305K before increasing again at higher temperatures. When salts are dissolved in the water, the curve changes, and in particular, the minimum appears at a different temperature, the position of the minimum depending on the concentration of the salt in solution.
Dillon and Dougherty found that the concentration at which the temperature minimum of specific heat is 298K - the temperature at which the NMR experiment was carried out - closely matches that at which the change in slope of the chemical shifts occurred. This was 2.4 mol per litre for KF (see Fig. 2), 1.6 mol per litre for KCL and 2.95 mol per litre for LiOH.
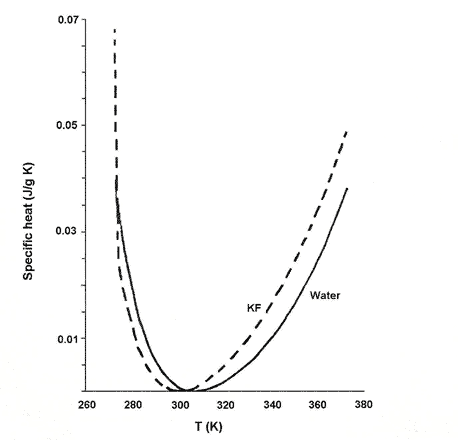
Figure 2. Change in specific heat of KF solution at 2.4 mol/l with temperature compared with pure water.
The specific heat capacity of the solution is its capacity to absorb heat energy, measured in energy units per gram per degree K increase in temperature. Plots of the specific heat capacity of electrolyte as a function of temperature are similar to the corresponding plot for pure water, but the perturbation of water structure by the electrolyte results in a shift in the location of the minimum (compared with pure water) as well as subtle changes in the shape of the curve. A correlation of the changes in slope of chemical shifts to minima in specific heat capacity suggests that there is a weak continuous phase transition (see Box 2) in the structure of the solution at the critical concentration corresponding to the specific heat capacity minimum. A phase-transition is a global phenomenon involving the entire solution.
Box 2
Phase transitions
Phase transitions refer to abrupt changes in the collective properties of all the molecules (phases), with a small change in a variable such as temperature; for example, when ice changes into water or water changes into gas and vice versa.
Phase transitions are classified into two broad categories. First order phase transitions are discontinuous, involving the absorption or release of a 'latent heat', a fixed amount of energy, as in the changes of water between the liquid and gas phases. Second order phase transitions are continuous phase transitions that have no associated latent heat. Examples are ferromagnetic transition and transition into superfluid state.
This global phase transition, involving the entire solution, can be explained by changes in water structure occurring as a result of changes in the hydrogen bond strength, due to changes in electrolyte concentration, and "electron delocalisation throughout the liquid". In other words, dissolving salts in water changes the structure of water globally as a whole
Could that interpretation apply to entire lakes and oceans? That's enough to send shivers up and down my spine.
These and other exciting results (see articles following) are likely to fuel the wide-ranging debates on water, from its dynamic structure at one extreme to the scientific basis of homeopathy and consciousness at the other.
Article first published 29/06/04
Comments are now closed for this article