New age of water
Water has come of age. It is cool on everyone's lips. Decades of research on water is giving us remarkable insights into its dynamic collective structure, and changing our big picture of life and living process.
Organisms are seventy to eighty percent water. Is this water necessary to life? What vital functions does it serve?
Entire biochemistry and cell biology textbooks are still being written without ever mentioning the role of water. It is simply treated as the inert medium in which all the specific biochemical reactions are being played out.
Instead, recent findings are raising the possibility that it is water that’s stage-managing the biochemical drama of life. Water is life, it is the key to every living activity. Some people will even say it is the seat of consciousness.
I-SIS brings you the latest revelations on water in this extended series that starts from the basics.
Water, the most abundant constituent of living organisms, is associated with an enormous amount of surfaces inside cells and in the extracellular matrix. Is all of this biological water different from water in bulk? The answer is definitely yes, if the incredible new findings are to be taken on board. Dr. Mae-Wan Ho reports
"Biological" water includes practically all the water in living organisms, inside the cell as well as in the extra-cellular matrix, except, possibly, for large reservoirs or conduits such as the bladder, gut, stomach and vacuoles inside some cells. Biological water is rarely far from the surface of a membrane or a macromolecule such as proteins, nucleic acids and polysaccharides like starch and glycogen.
Inside the cell, the concentration of proteins in cytoplasm is between 170 to 300 mg/ml, which suggests that 7 to 9 shells of water (hydration shells) coat the available surfaces, corresponding to a distance of 4 to 5nm (nanometre, 10-9m) between the surfaces. A substantial fraction of the water is quite closely associated (at a distance of less than 0.5nm) with the proteins, nucleic acids, polysaccharides and assemblies of smaller molecules that make up an organism, and is essential for their functioning.
The idea that cell water is distinct from bulk liquid water goes back a long way to pioneers like Gilbert Ling and Albert Szent-Györgyi in the 1960s and 70s; to many physicists and chemists in the latter half of the 19th century fascinated by the distinctive properties of 'protoplasm' inside living cells.
Since the 1970s, many physical and physiological techniques have demonstrated that cell water behaves very differently from bulk water. It is dynamically ordered or oriented, and exhibit restricted motion compared to water in the bulk.
More recently, ordered interfacial water have been found to be associated with pure protein or DNA crystals obtained at cryogenic (very low freezing) temperatures. These ordered water molecules do not form the typical ice structure, but are involved in many different forms of hydrogen bonding networks with the macromolecule and with each other.
A major uncertainty is what fraction of the water in living organisms and cells is distinct from bulk water, and to what extent water is essential for different living functions.
Using sophisticated techniques with big machines, such as NMR and more recently, neutron diffraction, no more than one or two layers can be detected to have altered properties, which would imply that a substantial part of the water inside cells and in the extracellular matrix is still bulk water.
But other scientists, notably, Gilbert Ling, who emigrated to the United States on a Boxer Fellowship from China, has been insisting since the 1960s that practically all the water in the cell is in an 'altered' state different from bulk water (see SiS review).
Water generally forms ordered layers over solid surfaces, and this ordered 'interfacial water' can tell us a great deal about water in living organisms.
Interfacial water has different properties from bulk water; for example, certain solutes that dissolve in bulk water are excluded from interfacial water, or fail to dissolve in it.
Interfacial water is generally thought to be no more than one or at most several layers of water molecules thick. But several reports published in the 1990s suggested that hydrophilic (water-loving) surfaces could extend their influence over much larger distances from the interface.
Gerald Pollack and Zheng Jian-ming in the Department of Bioengineering, University of Washington, Seattle in the United States decided to do some simple elegant experiments to find out exactly how far such hydrophilic surfaces can extend their influence; and came up with some startling results.
They used as solutes, microspheres 0.5 to 2 mm in diameter, which can be seen with the ordinary light microscope. For the hydrophilic surfaces, they employed several common hydrogels known to interact strongly with water.
In the first experiment they put a small gel sample between two large glass cover slips, and filled the space to either side with a suspension of the microspheres, then sealed the chamber. The whole assembly was placed on the stage of a microscope fitted with a camera to follow what happens.
In the second experiment, the gel was formed around a glass cylinder, which was withdraw after the gel was formed, leaving a channel, l mm in diameter, which is then filled with the suspension of microspheres and placed under the microscope.
To their amazement, they found that the microspheres were excluded from the gel surfaces in both experiments over distances of tens of mm, and in extreme cases, up to 250mm or more. Such massive exclusion zones are totally unexpected, and have never been reported before (see Fig. 1).
Microspheres were almost completely absent from the exclusion zone, and the boundary between exclusion and non-exclusion rather sharp, of the order of 10% of the width of the exclusion zone. The zone forms rather quickly, and appears 80% complete after 60s. Migration velocity was about 1.5mm per second, and microspheres near the boundary migrated at the same speed as those far away from it. Once formed, the exclusion zones remained stable for days.
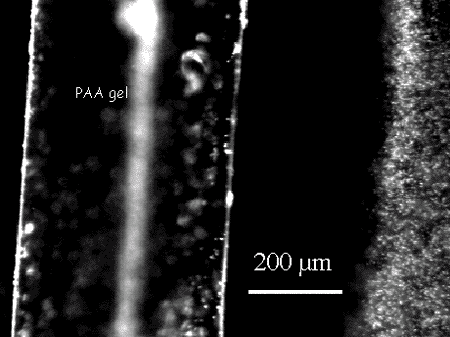
Figure 1. Exclusion zone formed next to the surface of polyacrylic acid gel.
Could this be an artefact? For example, could there be some invisible threads sticking out from the gel surface to push the microspheres away? They tested this by using the atomic force microscope and other sensitive probes to detect such strands, but no protruding strands were detectable, not even after they fixed and cross-linked the gel and washed it extensively, so no lose strands could ever leak out.
Could it be that the gel was in fact shrinking away from the surface and extruding water, and therefore squirting the microspheres away? But no such shrinkage was detectable; the boundary did not shift appreciably as the microspheres migrated away from it. Over a period of 120 minutes, the diameter of the cylindrical hollow in the gel changed by less than 2mm. Thus, in the 2 min period during which the exclusion zone was formed, shrinkage was insignificant.
Could it be that polymers were leaking out into the exclusion zone, and pushing away the microspheres? They added a polymer to the microsphere suspension, but this only narrowed the exclusion zone.
Yet another test was to continuously infuse microsphere suspension into the cylindrical hollow in the gel under pressure at a speed of about 100mm/s, so that any suspended invisible solutes ought to be washed out. But the exclusion zones persisted, virtually unchanged even at the highest speeds.
The exclusion zones were not a quirk due to the particular gel used. Polyvinyl alcohol gel, polyacrylamide gels, polyacrylic acid gels, and even a bundle of rabbit muscle all gave similar results (Fig. 2); and microspheres of different dimensions, coated with chemicals of opposite charge nevertheless resulted in exclusion zones. Thus, exclusion zones are a general feature of hydrophilic surfaces. One gel that did not show exclusion was when polyacrylamide was copolymerised with a vinyl derivative of malachite green.

Figure 2. Exclusion zone next to surface of rabbit muscle.
Exclusion was most profound when the microspheres were most highly charged, so negatively charged microspheres gave maximum exclusion at high pH, whereas positively charged microspheres gave maximum exclusion at low pH. The presence of salt tended to decrease the size of the exclusion zone somewhat. The size of the exclusion zone also went up with the diameter of the microsphere.
What could be the explanation for this strange phenomenon that has never been observed; that apparently goes against all expectations based on data from the latest big machines?
After ruling out several trivial explanations, Zheng and Pollack considered whether it could be due to layers of water molecules growing in an organized manner from the gel surface and extending outwards, pushing the microspheres out at the same time. That would seem consistent with the observation that the speed of migration of the microspheres is constant regardless of distance from the boundary. It is also consistent with the finding that larger microspheres give bigger exclusion zones.
The increase in exclusion zone with charge, too, is consistent with their water-structuring hypothesis, as higher surface charge is known to be associated with larger extent of water structuring. But, as they remark, "While these several observations fit the water-structure mechanism, no reports we know of confirm any more than several hundred layers of water structure at the extreme, and not the 106 solvent layers implied here."
Zheng J-M, and Pollack GH. Long-range forces extending from polymer-gel surfaces. Physical Review E 2003, 68, 031408.
Article first published 30/06/04
Comments are now closed for this article