Water charges up with electricity when exposed to sunlight, offering the potential for an inexhaustible supply of squeaking clean energy and challenging conventional understanding of bioenergetics. Dr. Mae-Wan Ho
Put some water next to any hydrophilic (water-loving) surface and expose it to sunlight, or even light from an ordinary light bulb, and the water will charge up with electricity all by itself. This is the latest in a series of extraordinary discoveries about water from the laboratory of US bioengineer Gerald Pollack at the University of Washington in Seattle.
It began when Pollack and his student Zheng Jian-ming discovered that suspensions of colloids and dissolved substances are excluded from a region extending some hundreds of micrometres from the surfaces of hydrophilic gels [1] (Water Forms Massive Exclusion Zones, SiS 23). An ‘exclusion zone’ (EZ) of this magnitude is in direct contradiction to the generally held assumption that interfacial water forming at liquid-solid, or liquid-air interfaces can be no more than a few layers of molecules thick. Instead, what’s observed is a million layers or more.
Similar exclusion zones were found next to any hydrophilic surface including surfaces coated with a monolayer of hydrophilic molecules, and around ion exchange resin beads [2] (see Fig. 1). Electric charge appears to be important, as EZ failed to form around charge-exhausted resin beads. Although EZ can form in pure water, it is enhanced and stabilized by low concentrations of buffer (2 to 10 mM at pH 7).
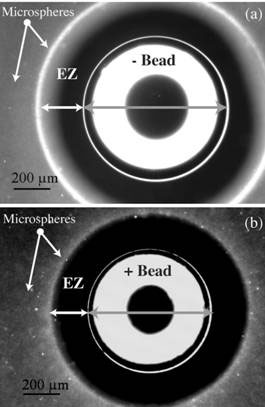
Figure 1. Exclusion zones millions of layers of water molecules deep clear of suspended microspheres form around charged resin beads
The EZ was characterized by several spectroscopic methods, all of which showed that it had features very different from the bulk water, suggesting an unusually ordered crystalline phase where the molecules are less free to move [3, 4] (Liquid Crystalline Water at the Interface, SiS 38). The UV and visible absorption spectrum gave a single absorption peak at ~270 nm in the UV region, which is completely absent in the bulk phase. The infrared emission record showed that the EZ radiates very little compared with bulk water, as would be expected on account of the reduced mobility of water molecules. The magnetic resonance imaging mapping similarly gave a transverse relaxation time (T2) of 25.4 + 1 ms, which is shorter than the 27.1 + 0.4 ms recorded for the bulk water phase, again indicative of restricted motion.
Such coexistence of distinctly different phases has been demonstrated in 1999 in by Japanese water researcher Norio Ise and colleagues in Kyoto University [5] (Water and Colloid Crystals, SiS 32) using a dispersion of colloid latex particles in water and digital video recording. They captured a random phase, in which thermal motion of the particles is of the anticipated magnitude, right next to a crystal-like phase where the particles had separated regularly from one another by several micrometres and the deviations from their average positions are lower by an order of magnitude
Most surprisingly, Pollack and colleagues discovered that the EZ had a different electrical potential from the bulk phase, by as much as 100 – 200 mV [6], depending on the hydrophilic surface. With a negatively charged surface such as polyacrylic acid or Nafion (widely used as a proton exchange membrane), the potential is negative compared with the bulk water away from the EZ. Simultaneously, the hydrogen ion (proton, H+) concentration is high just outside the EZ, decreasing in a gradient away from it [4]. This clearly indicates that the formation of the EZ is accompanied by a separation of positive and negative electrical charges, which led to the build up of electrical potential between the EZ and the bulk water. In effect, the water has become an electrical battery, and can provide electricity through an external circuit.
Separating H+ from e- (electron) is the first step of photosynthesis in green plants which provides energy for most of the biosphere [7] (see Harvesting Energy from Sunlight with Artificial Photosynthesis, SiS 43). But where does the energy come from in the case of EZ? It turns out to have more in common with photosynthesis.
A clue came after having inadvertently left the experimental chamber with the EZ on the microscope overnight. Next morning, the EZ had shrunk considerably. But after turning on the microscope lamp, it began to immediately grow again, restoring itself within minutes to its former size. The energy for EZ formation comes from light, as in photosynthesis, but it can use the low energy part of the solar spectrum that photosynthesis cannot.
Although the entire spectrum of visible light appeared effective in making the EZ grow, the most effective part is in the infrared region, peaking at ~3 100 nm. A 10 minute exposure at that wavelength expanded the width of an EZ 3.7 times, and after an hour of exposure, the expansion was more than 6 times [8].
After the light was turned off, the EZ remained constant for about 30 minutes before beginning to shrink, reaching halfway to its baseline level in about 15 minutes.
When the UV and visible range was tested, a peak in the degree of EZ expansion was detected at 270 nm in the UV region, corresponding to the characteristic absorption peak of EZ that was identified before. However, as the optical power used in the UV and visible region was 600 times that in the IR, the most profound effect was identified in the IR region, particularly at 3 100 nm.
The mechanism of EZ formation is still unknown. But the two wavelengths that expand the EZ most effectively may offer some hint. The UV 270 nm is close to the 250 nm (~5 eV) required to ionize water under standard state conditions and taking into account the hydration of the resulting ions [9]. The 3 100 nm peak, on the other hand is close to the OH stretch of the ring hexamer identified as the most abundant species in infrared predissociation spectroscopy of large water clusters [10], and also in neon matrices by infrared spectroscopy [11]. These results suggest that photoexcitation of ring hexamers and photoionisation followed by ejection of protons play synergistic roles in the assembly of the EZ phase. Pollack and colleagues believe that the infrared radiation, though normally insufficient to break OH bonds, can nevertheless work via resonance induced dissociation of large hydrogen-bonded networks.
What do these findings mean outside the lab? The 3 100 nm IR source is about 0.6 percent of the sun’s overall energy, which is ~8.4 W/m2. By comparison, the power density of the LED light source used in the lab was 1.2 mW/m2, almost seven thousand times lower. Chai Binghua, Yoo Hyok and Pollack speculate that nature may contain a whole lot more EZ water than most people think. In other words, an appreciable fraction of the sun’s energy may be stored as charged EZ water. What this means for aquatic life is a large open question.
The earth is known to have a large negative surface charge, resulting in an electric field on the order of 100V/m at the earth’s surface. Perhaps this arises from the earth’s surface water under the influence of radiant energy from the sun.
Finally, the widespread occurrence of EZ within living cells and tissues is bound to have a drastic effect on bioenergetics. After all, organisms are energized by nothing more than the exquisitely orchestrated flows of electrons and protons that enable them to do everything it means to be alive [10] (see The Rainbow and the Worm, The Physics of Organisms, I-SIS publication).
Article first published 25/06/09
Comments are now closed for this article
There are 9 comments on this article.
Anne Hooper Comment left 6th July 2009 00:12:39
I am not a scientist, but your discoveries are wonderful. Keep on with your work. Don't allow the 'powers that control the peoples of this world' to stop your research.
tony villar Comment left 27th June 2009 23:11:19
pls go on. this is great.
mae-wan ho Comment left 27th June 2009 23:11:33
reply to james S lee: Na+ and Cl- ions naturally separate in bulk water and that does not provide by itself a method of desalination.
James S Lee Comment left 25th June 2009 12:12:19
Your statement, "...the formation of the EZ is accompanied by a separation of positive and negative electrical charges, which led to the build up of electrical potential between the EZ and the bulk water", made me wonder if the same mechanism can lead to the separation of Na+ and Cl- ions in sea water, thus possibly providing another method of desalination?
Sue Marriott Comment left 2nd November 2009 01:01:14
I am so excited by this whole concept... which makes sense at a gut level to this non-scientist. I really want to iron out my understanding and fill gaps in my knowledge... sometimes at a very basic level... with this in mind I have just started a Yahoo group entitled EZ Water.. if anyone wants to join and discuss. Thanks.
http://tech.groups.yahoo.com/group/EZWater/
jozzy-online Comment left 2nd March 2010 19:07:56
tres interessant, merci
James Vega Comment left 13th April 2011 23:11:16
Can the charging of water be increased with metal colloids added to the water like silver? Also what if there are other colloids or particles in the water? Would these effect the formation EZ or the behavior of it?
RG Comment left 3rd January 2012 00:12:39
May I suggest dr. Matveev's review of Pollack's work in light of Ling's research: http://www.bioparadigma.spb.ru/files/Matveev-2002-Revolution.and.counter.revolution.pdf
Camelopardalus Comment left 30th July 2012 22:10:57
Vladimir Matveev's critique is valid, but not really pertinent to the EZ work mentioned in this article.
The Water EZ is a physical phenomenon, exhibiting a first-order phase transition and a self-organizing system. It is much more fundamental and groundbreaking than the earlier book; the work Dr. Pollack and his team have accomplished exploring the EZ stands on its own and has been extensively peer-reviewed and repeated.