The unique complexities of liquid water make it anomalously fit for life. Prof Martin Chaplin
Everyone knows some of the properties of liquid water. Often they think of these properties as typical of liquids in general; for example, most people believe all liquids dissolve gasses less well at higher temperatures. This is a mistake as the opposite is true. Liquid water only behaves like most other liquids at very high temperatures (i.e. when superheated) but is strange and atypical at low temperatures. Overall, liquid water can be considered an intimate mixture of two miscible phases, one predominant at lower temperatures and the other predominant at higher temperatures. Hydrogen bonding is generally said to be the cause of these phenomena but confusion still exists over what ‘hydrogen bonding’ in water entails. No longer should we simply describe (or model) liquid water in terms of individual water (H2O) molecules or describe water’s hydrogen bond as simple electrostatic interactions between discrete molecules. We must consider both proton quantum effects and extensive electron delocalization within network(s) of water molecules (i.e. neither the protons nor electrons are pinned to individual molecules).
Organisms consist mostly of liquid water and life on Earth depends on the unusual structure and anomalous nature of liquid water [1]. Biological water performs many functions and should never be considered simply an inert diluent; it transports, lubricates, reacts, stabilizes, signals, structures and partitions. The living world should be thought of as an equal partnership between the biological molecules and water. The middling strength of the connecting hydrogen bonds between water molecules seems ideally suited to life processes, being easily formed but not too difficult to break. In spite of much work, many of the properties of water remain puzzling.
The high cohesion between molecules endows water with a high freezing and melting point, such that both we and our planet are bathed in liquid water. Water’s large heat capacity and high thermal conductivity prevent local temperature fluctuations in organisms, enabling them to more easily control their body temperature. The large latent heat of evaporation gives resistance to dehydration and considerable evaporative cooling. Singularly, water ionizes and allows easy proton exchange between molecules, so contributing to the richness of the ionic interactions in biology. Liquid water is an excellent solvent due to its polar nature, high dielectric constant and small size, particularly for polar and ionic compounds and salts. It even dissolves glass and plasticisers; and it is, therefore, very difficult to obtain really pure water containing less than 5 ng g-1 solute. Water’s unique hydration of the important biological macromolecules - proteins and nucleic acids - determine their three-dimensional structures, and hence their functions. Similarly, liquid water interacts with smaller molecules, giving rise to complex structural transformations, aggregations, and differentiable aqueous phases at both macroscopic and nanoscopic scales. Hydration leads to the formation of sols and gels; reversible gel-sol phase transitions underlie many cellular mechanisms.
At close to 4 °C, water expands on both heating and cooling. This density maximum, together with the low ice density results in (i) the necessity that all of a body of fresh water, and not just its surface, is close to 4 °C before any freezing can occur; (ii) the freezing of rivers, lakes and oceans from the top down, so permitting survival of the bottom ecology, insulating the water from further freezing, reflecting back sunlight into space, and allowing rapid thawing; and (iii) the density-driven thermal-convection that results in seasonal mixing and oxygenation of the depths.
The large heat capacity of the oceans and seas allows them to act as heat reservoirs such that sea temperatures vary only a third as much as land temperatures, thereby moderating our climate. Water's high surface tension plus its expansion on freezing encourages the erosion of rocks to give soil for our agriculture.
Notable amongst the anomalies of water are the diametrically opposite properties of hot and cold water, with the anomalous behaviour more accentuated at low temperatures where the properties of supercooled water also often diverge from those of hexagonal ice as the temperature is lowered further. As very cold or supercooled liquid water is heated it contracts and becomes less easy to compress, its refractive index increases, the speed of sound within it increases, gases become less soluble and it is easier to heat and conducts heat better. In contrast as hot liquid water is heated it expands, it becomes easier to compress, its refractive index decreases, the speed of sound within it diminishes, gases become more soluble and it is harder to heat and is a poorer conductor of heat. With increasing pressure, cold water molecules move faster but hot water molecules move slower. Hot water freezes faster than cold water and ice melts when compressed except at high pressures when liquid water freezes when compressed. No other material is commonly found as solid, liquid as well as gas on Earth. Insight into these anomalous properties of water comes from the understanding that water molecules form an infinite hydrogen-bonded network with two forms of localized and structured clustering.
Liquid water is best described as an intimate mixture of two liquid phases: one that has good three dimensional hydrogen bonding, an open and less dense structure and with lower hydration capability; and one that consists mostly of hydrogen-bonded chains, with a denser, more compact structure and greater hydration capability. The balance between these two structural forms is dependent on the temperature, pressure, solutes and other phases present in a way that is easy to understand once the basic concept is appreciated.
An important fact, recently discovered and often overlooked, is that liquid water is not homogeneous at the nanoscopic (i.e. nanometre) level [1-7]. The high density of liquid water is due mainly to the cohesive nature of its hydrogen-bonded network, with each water molecule capable of forming four hydrogen bonds. This reduces the free volume and ensures its relatively high density, partially compensating for the open nature of the hydrogen-bonded network. It is usual for liquids to expand when heated, at all temperatures. The anomalous temperature-density behaviour of water can be explained utilizing the range of environments within whole or partially formed clusters. The density maximum at 4 °C is brought about by the opposing effects of increasing temperature, causing both (i) structural collapse of the hydrogen bonding so increasing density and (ii) thermal expansion so lowering density.
Counter-intuitively, the distance between the water molecules decreases as the density decreases at lowered (supercooled) temperatures; the decrease in density being primarily due to the reduction in the number of nearest neighbours. At lower temperatures there is a higher concentration of expanded clusters caused by stronger, more directional hydrogen bonds. However, at higher temperatures there is more collapsed hydrogen bonded clustering and smaller hydrogen-bonded clusters, but the volume they occupy expands with temperature due to the increased molecular kinetic energy. The change to less extensive hydrogen-bonded clusters in the liquid water as the temperature rises is accompanied by positive changes in both entropy and enthalpy due to the less ordered structure and greater hydrogen bond bending respectively (Figure 1).
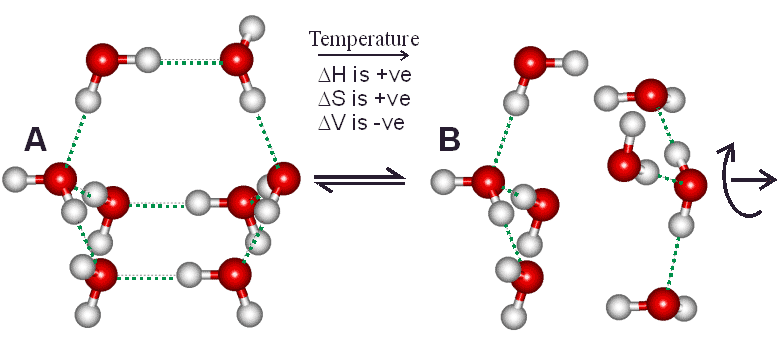
Figure 1 Two water tetramer clusters may form from an octamer cluster
Small clusters of four water molecules (B) may come together to form bicyclo-octamers (A). Structures similar to (B) have greater numbers of 2-, 3- and 5-coordinated water molecules as found at higher temperatures in liquid water, whereas structures similar to (A) have greater numbers of 4-hydrogen bonded and 4-coordinated water molecules as found at lower temperatures in liquid water. Equilibrium between (A) and (B) is finely balanced as there are two potential energy minima due to the differing entropy compensation of hydrogen bonding and van der Waals interactions; with the stronger hydrogen bonding (but lower entropy) (A) structure being more stable at lower temperatures.
Competition between maximizing van-der Waals interactions (B, yielding higher orientation entropy, higher density and individually weaker but more numerous water-water binding energies) and maximizing hydrogen bonding (A, yielding more ordered structuring, lower density and fewer but stronger water-water binding energies) is finely balanced, and easily shifted as physical conditions, solutes and surfaces change. The potential energy barrier between these states ensures that water molecules prefer either structure A or B with little time spent on intermediate structures. An individual water molecule may be in state A with respect to some neighbours while being in state B with respect to others, resulting in density fluctuations at the nanometre scale in liquid water. Moreover, these bicyclo-octamers may cluster together to form more extensive networks, stabilizing clathrate cavities; and these larger three-dimensional clusters will be more evident at even lower temperatures (Fig. 2). Many papers have now been published showing the presence of this 'two-state' clustering in liquid water and how this concept helps in understanding the properties of liquid water and its solutions.
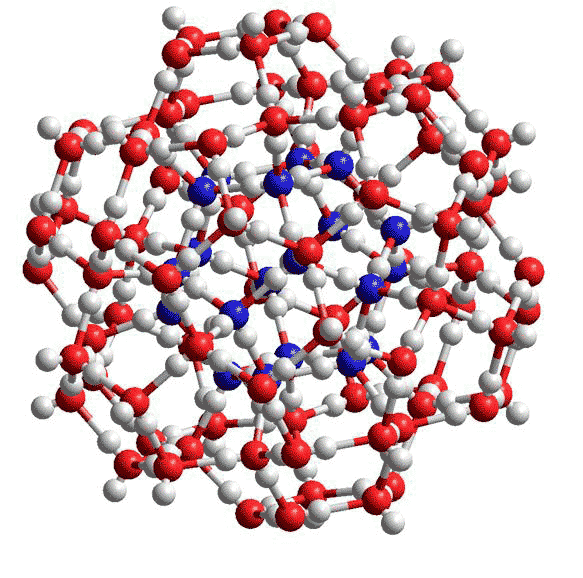
Figure 2 The smallest water cluster that may be stabilized by small hydrophobic molecules or kosmotropic ions is the (H2O)100 cluster as found in the cavity-encapsulated nanodrop of water in a polyoxomolybdate [8]. In this diagram, the oxygen atoms in the central (H2O)20 dodecahedron are coloured blue
The hydrogen bonding, although cohesive in nature, is thus holding the water molecules apart. It is the conflict between these two effects, and how it varies with conditions, which endows water with many of its unusual properties. Solutes will interfere with the cluster equilibrium by favouring either open (such as A) or collapsed (such as B) structures. Any such effect will cause the physical properties of the solution, such as density, viscosity or solubility of co-solutes, to change.
Clusters linked by extensive hydrogen bonding can be considered connected by extensive electron delocalization (Fig. 3). As electrons are not held by individual molecules but are easily distributed among water clusters they can give rise to coherent regions [9] interacting with local electromagnetic radiation. The water protons are also not held by individual molecules but may switch partners in an ordered manner within distinct networks.
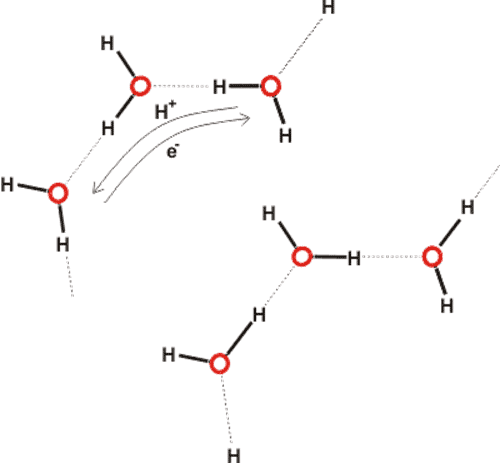
Figure 3 Neither the protons nor the electrons are pinned to individual molecules
Atmospheric gases dissolve in water and then form nanobubbles (< 1 micron diameter) and microbubbles (1-100 micron diameter) some of which may expand and rise back to the surface. This process causes continuous, but somewhat chaotic, heterogeneous changes in the gaseous concentrations in liquid water over significant time periods (» 100 s) and consequently continuous changes in the hydrogen-bonded structuring and functional properties within the water.
Nanobubbles are gas-containing cavities in aqueous solution [10-12]. They are under excess pressure as the surface tension causes a tendency to minimize their surface area, and hence volume. The gas in nanobubbles is in constant flux with the nanobubbles growing or shrinking by diffusion according to whether the surrounding solution is over-saturated or under-saturated with the dissolved gas relative to the raised cavity pressure. As the solubility of gas is proportional to the gas pressure and this pressure is exerted by the surface tension in inverse proportion to the diameter of the bubbles, there is increasing tendency for gasses to dissolve as the bubbles reduce in size. The internal gas pressure increases greatly at very small bubble diameters and so accelerating the process.
Such dissolution is increased by the bubble's movement and contraction during this process, which aids the removal of any gas-saturated solution around the cavities. Theoretical calculations, using the well-established Laplace equation, show that nanobubbles should only persist for a few microseconds [13]. However, the ease with which water forms larger visible bubbles under slight tensile pressure well below the tensile strength of water and the greater difficulty of degassing, both indicate the widespread occurrence of gas-containing nanobubbles (cavities). During recent years, many workers have shown that such nanobubbles (diameter about 100 nm) are commonly found together with nanoparticles of solids or highly hydrated semisolids although such solutions appear clear. Bulk-phase nanobubbles can be detected by light scattering or resonant mass measurement and easily distinguished from nanoparticles. Clusters of nanobubbles have been proposed that are stabilized by ionic solutes and containing gas at atmospheric pressure [14]. A high density of nanobubbles may be created in solution with the heterogeneous mixture lasting for weeks [10]. The total amount of gases in these nanobubble solutions may reach 600 cm3 per litre of water (volume converted to standard temperature and pressure). This enables poorly soluble substances to be stably suspended in solution, such as flavours that are easily recognized by taste.
The most likely reason for the long-lived nanobubbles is that the nanobubble gas/liquid interface is charged [15], introducing an opposing force to the surface tension, so slowing or preventing their dissipation. Thus, although the surface tension by itself would cause the bubble surface to shrink, this is prevented by repulsion between the charges in the surface, which by themselves would cause the surface to expand. It is clear that the presence of like charges at the interfaces reduce the effect of surface tension, with charge repulsion acting in the opposite direction to the surface tension. Any effect may be increased by the presence of additional charged materials that favour the gas-liquid interface, such as the natural concentration of hydroxide ions at neutral or basic pH. It is further probable that the surface charges are stabilized by the higher concentration of dissolved gas in the surface layer, which produces an environment favourable to large chaotropic anions. Such negatively-charged surfaces are extensive and will have their own solubilization characteristics, being able to hold molecules that are otherwise extremely poorly soluble in bulk liquid water. In addition, bubble stability is greater due to the slow rate of gas diffusion to the bulk liquid surface from nanobubbles [16].
Nanobubbles have a tendency towards self-organization [17] in much the same way as charged oil-water emulsions, colloids [18] and nanoparticles. This is due to their charge, long range interactions and slow diffusion. Where there are large numbers of bulk phase nanobubbles, such as in electrolyzed aqueous solutions, there is relatively large amounts of water associated with the surfaces, which have different properties to bulk water.
The recent explosion of interest in aqueous nanobubbles is largely on account of the extensive and surprisingly stable gas-liquid interface they create, which enables them to solubilize and suspend hydrophobic materials without requiring detergents [19-21]. Furthermore, they are widespread in biological and natural waters, and may contribute to various puzzling biological phenomena.
The generally accepted view of osmotic pressure is that it is a colligative property – one that depends on the concentration of solutes - along with freezing point depression, boiling point elevation and vapour pressure lowering. Osmotic pressure, however, is also generated, without any solute, at hydrophilic surfaces. It is, perhaps, unsurprising that ion exchange surfaces can generate very high osmotic pressures of over 100 MPa in their surrounding water [22], as they create high surface concentrations of counter-ions. Poly-ionic nanoparticles, with high surface area, produce such a great osmotic pressure that they can be used in practical desalination processes [23, 24]. However, it has been experimentally verified that uncharged hydrophilic surfaces can do this also, without the presence of counter-ions or solutes [25, 26]. Much work on the effect of hydrophilic surfaces on the mesoscopic properties of the adjoining aqueous solutions has been done in the laboratory of Gerald Pollack [27, 28], and the phenomena have been confirmed by many other independent workers [29-31]. In essence, it has been found that the interfacial water next to ionic charged or neutral uncharged hydrophilic surfaces expels solutes to the bulk of the solution that may be several hundred microns away. These exclusion zones (named as EZ-water) can be visualized when low-molecular weight dyes, proteins, micron-sized microspheres or other solutes are used. With a laser tweezers system, force fields inside the solute-free exclusion zones have been found to diminish as a function of distance from the surface [32]. Also the EZ-water seems to possess other physical properties such as absorption at 270 nm [33], greater density, greater viscosity and negative charge compared with the bulk water.
Wherever water is present in solution it may be considered as being either 'bound' or 'free', although there will be transitional water between these states. In the case of colligative properties, water is considered bound to any solute when it has a lower entropy compared with pure liquid water. Such water may be considered part of the solute and not part of the dissolving 'free' water. As pure liquid water consists of a mixture containing low-density water, made up of extensively hydrogen bonded structures (Figure 1 A), and higher density water (Figure 1 B), consisting of much smaller less extensive clusters, the proportions of 'bound' or 'free' water in pure liquid water can vary, with the more strongly-bound larger clusters behaving more like 'bound' water. In bulk liquid water, the relative concentrations of the two aqueous forms is of no consequence as all the water behaves the same throughout. If volumes of the solution contain different proportions of strongly and weakly hydrogen-bonded water molecules (or put even more simply that there is more extensive clustering present), then these different volumes will show a difference with respect to their water activity and chemical potential. Normally any such instantaneous differences in water activity and chemical potential between different volumes within the same mass of liquid would rapidly cause liquid movement from one to the other in order to equalize these states and so remove the chemical potential differences. However, where there are surfaces interacting with the liquid water, the concentration of the more extensive hydrogen-bonded clusters within the surface layer may differ from the bulk values with the surface interactions preventing the potential equalization between bulk and surface volumes. When this occurs, the surface water has a different water activity and chemical potential to the bulk, leading to differences in osmotic pressure, and other colligative properties. The change in the chemical potential (μw) is –{RTLn(xws) -RTLn(xwb)} (that is, a negative energy term is added to the chemical potential when xws < xwb) where xws is the mole fraction of the ‘free’ water (0 < xws < 1) in the surface layer and xwb is the mole fraction of the ‘free’ water (0 < xwb < 1) in the bulk liquid.
At hydrophilic surfaces, interactions between the surface and neighbouring water molecules fix the localised hydrogen bonding and this, together with steric factors, increases the cluster extent and lifetime [34]. As the ‘free’ water is reduced by the formation of longer-lived and more extensive hydrogen bonded clusters [35], so the osmotic pressure increases. This increase in osmotic pressure next to the surface will displace solutes from the surface towards the bulk until its effect is equalled by the osmotic pressure of the solution or the system reaches a steady state. As the first effect of this solute expulsion is naturally the formation of an increased concentration band as expelled solute mixes with the prior solute concentration, the extent of the expulsion will affect the whole of the unstirred layer (~1-100 µm). Where hydrophilic microparticles or nanoparticles are suspended, their surfaces will necessarily cause mutually repulsive osmotic pressure effects that may result in the ordering of the particles within small volumes of the liquid [36, 37]. It should be noted that osmotic drive does not require a membrane to separate the two solutions [38] provided there are two phases. Here the two phases consist of the unstirred and stirred layers. In this context, the affected aqueous layer behaves similarly to that described for exclusion zone (EZ) water by Pollack and this is put forward as a simple explanation of his experimental data [28, 33, 39]. It also shows similarities with the experiments on autothixotropy [40-44], where the viscosity of unstirred solutions increase with time. The increase in density at the interface, as found in EZ-water, has been explained previously by the increase in clustering causing the water to behave as though it is at a lower temperature, which has also been used to explain the ease with which this surface layer freezes. The presence of 270 nm absorption in the interfacial water, as described for EZ-water [33],30 may be attributed to the delocalization of electrons within the extended clustering as hydrogen bonding is known to involve electron delocalisation [45].These electron delocalizations are stabilised by the addition of electrons but not by protonation, so causing the charge separation seen at these interfaces [46].
Another effect of interfaces is the formation of evanescent waves due to the internal reflection of electromagnetic radiation. The standing electromagnetic wave produced will interact with water molecules to stabilise a standing wave of hydrogen bonded clusters that will increase the local concentration and extent of hydrogen bonded clusters so increasing the above osmotic effect, in agreement with the experimental data [47-50].
Liquid water should not be thought of as a simple solvent. Its properties are unlike those of most liquids in that it behaves as an intimate mixture of clusters with differing properties.
Article first published 18/03/13
Comments are now closed for this article
There are 3 comments on this article.
Hernando Durana Comment left 16th March 2015 07:07:50
Most interesting article. Water in universe a miracle compound.
Hernando Durana Comment left 26th November 2016 22:10:23
Do you have this article in Spanish?
Thanks
Emma Aiken Comment left 19th September 2015 06:06:32
I can't pretend to understand all the science in this article, but what stands out to me are the references to the opposing tendencies in water, e.g. the opposing effects of increasing temperature, and the diametrically opposite properties of hot and cold water. Liquid water appears to have dialectical dynamics, existing on the edge of order and chaos, as do all living systems