Not just base metals into gold; but the profuse creation of elements that is rewriting the book of genesis. Dr. Mae-Wan Ho
Cold fusion scientists have managed, not so much to transmute base metals into gold (although there have been unconfirmed reports to that effect), but more spectacularly, to make a whole range of elements on the lab bench, with equipment not much more sophisticated than what the ancient alchemists might have used. In the process, nuclear energy is released - safely and without toxic or radioactive wastes - that could be harnessed for heating or to generate electricity [1] (see From Cold Fusion to Condensed Matter Nuclear Science, SiS 36).
In addition, there is the attractive possibility of solving the world’s nuclear waste problem (see Box) by transmuting highly radioactive and toxic nuclear wastes from conventional nuclear reactors into safer non-radioactive elements [2].
The most pressing nuclear waste problem is the high level radioactive waste produced by nuclear reactors. It contains nuclear fission products and transuranic elements (with atomic numbers greater than uranium) generated in the reactor core, which have half-lives greater than 20 years, in some cases thousands, or tens of thousands of years [3].
The US Environment Protection Agency recognizes the ionising radiation from nuclear wastes as a serious health hazard [4]. Acute exposures result in radiation sickness, burns, premature aging, or even death. Cancers and birth defects result from stochastic exposure. Some radioactive waste elements, such as U-238, are both radioactive and highly toxic. U-238 has a half-life of 4.5 billion years.
Nuclear wastes also constitute a major security concern, as they could be acquired by terrorist organisations or rogue nations and turned into nuclear weapons.
It is estimated that high level nuclear wastes is currently increasing by about 12 000 tonnes every year. Most of this waste is put into long-term storage after complicated treatments such as converting into glass or various concrete blocks. However, finding long-term storage sites that are safe and geologically stable remain a hot political issue in most countries.
Transmutation reactions come in two classes [5, 6]. The first class of reactions result in a large array of products with mass numbers spanning across the periodic table; these may involve the formation of a heavy compound nucleus that can decay and split into different elements (but see later). The second class of reactions give distinct, isolated products directly, without the compound nucleus intermediate.
These ‘cold’ or low energy transmutation reactions are remarkably easy to accomplish compared to the conventional ‘hot’ nuclear reactions that are supposed to take place in stars or supernova explosions, or else only at millions of degrees K.
By 2003, transmutation experiments have been studied in some detail by over 14 separate laboratories worldwide: Beijing University and Tsinghua University in China; Lab des Sciences Nucleaire in France; Frascati Laboratory and University of Leece in Italy; Hokkaido University, Mitsubishi Corporation, Osaka university, and Shizuoka University in Japan; SIA LUTCH, Tomsk Polytechnical University in Russia; Portland University USA, Texas A & M University, and University of Illinois Urbana-Champaign in the USA [5].
The minimum requirement for transmutation is a metal hydride film or membrane loaded up with hydrogen or deuterium to a high level, and kept in constant flux [5-8]. Electrode materials have ranged from carbon, nickel, to uranium. The metal hydride can be loaded by electrolysis of water or heavy water using a thin film of the metal as cathode; or else deuterium gas can be made to diffuse through the metal membrane by injecting the gas on one side and evacuating from the other side [9]. But a wide variety of experimental conditions have been used to trigger or speed up the reactions, including surface plasma electrolysis, plasma discharge, laser initiation and external electric or magnetic fields.
George Miley’s team at the University of Illinois Urbana-Champaign in the United States is one of the main groups involved in transmutation [5]. They used multi-layer thin film nickel, palladium or titanium [6] coated by sputtering on polystyrene microspheres, and loaded up to a high level of hydrogen by packing the coated beads in the cathode of an electrolytic cell. The products of nuclear reaction were documented carefully with a combination of secondary ion mass spectrometry (SIMS) and neutron activation analysis (NAA). SIMS detects most isotopes and is very sensitive but covers only a small area, typically a single microsphere, and is not very accurate. NAA on the other hand gives very accurate analysis of the entire electrode, but is restricted to detecting only certain elements. A combination of the two methods enabled the team to study a large number of isotopes. An overlap in the data set allowed a more accurate re-standardisation of the SIMS data to the more accurate NAA measurements.
A typical experiment is run continuously for 260 hours, resulting in a wide variety of elements. There are four high yield peaks in the atomic mass of 22-23, 50-80, 103-120 and 200-210. This pattern is generally consistent with results obtained by other research groups. Non-natural isotope distributions have been found for some elements, which is also a sign of nuclear reactions.
The most commonly reported elements are calcium, copper, zinc and iron. They were found in more than 20 different experiments. Forty percent of the least frequently observed elements were rare earths from the lanthanide group: lutetium, terbium praseodymium, europium, samarium, gadolinium, dysprosium, holmium, neodymium and ytterbium.
There were other effects associated with nuclear transmutation. These include energetic charged particles, protons (~1.6 MeV) and alpha (~16 MeV) emissions, and low level soft X-ray emissions. Excess heat was also produced simultaneously. Based on binding energy calculation, Miley concluded that the rate of transmutation correlates well with the excess power produced.
Transmutations have been obtained with both light and heavy water solutions, but heavy water appears to give a larger number of transmutation products under some conditions.
Yasuhiro Iwamura and colleagues at Mitsubishi’s Advanced Technology Research Center and colleagues have taken another approach to nuclear transmutation by concentrating on the direct transmutation of one element into another [10, 11].
They used D2 gas permeation through a sandwich of thin alternating layers of palladium (Pd) and CaO sitting on a bottom layer of bulk Pd. Permeation of deuterium is forced through the layers by exposing the top of the sandwich with a thin Pd film to D2 gas while the bottom is maintained under vacuum. On the D2 gas side, dissociative absorption causes the D2 molecules to separate into D atoms, which diffuse though the sandwich towards the vacuum side, where they emerge from the Pd metal, combine and are released as D2 gas (see Fig. 1). The element to be transmuted is deposited on the top Pd film of the Pd/CaO sandwich by electrolytic loading from a salt solution. Cesium (Cs), barium (Ba) and strontium (Sr) have been transmuted in this way. The analysis of elements was done in situ, without removing or disturbing the sandwich, using X-ray photoemission spectroscopy (XPS) directed at the topside of the sandwich
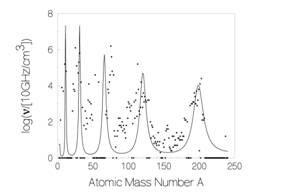
Figure 1. Transmutation by permeation (see text)
A typical experiment lasts for about a week or two. Cs has been transmuted into praseodymium (Pr) reproducibly in more than 60 experiments. Sr was transmuted into molybdenum (Mo) in three experiments lasting two weeks, the resulting Mo differed in isotope composition from natural Mo.
Based on an analysis of the depth profile of Pr, it appears that a very thin surface region of up to 10 nanometres is the active transmutation zone.
In the experiment involving transmutation of Ba to Sm, different isotopes of Ba resulted in the correspondingly different isotopes of Sm. 138Ba transmuted into 150Sm, and 137Ba transmuted into 149Sm, the increase in atomic mass was 12 in both cases, and atomic number 6. In both the transmutation of Cs to Pr and Sr to Mo, the increase in atomic mass was 8, and atomic number 4.
The role of the CaO layer was revealed in an experiment in which Cs was transmuted to Pr [10]. In all three samples with the normal Pd/CaO sandwich, Pr was found as the end product, but not in an experiment without a CaO layer; nor in two experiments in which the CaO layer was replaced by MgO. The CaO layer appeared to increase the deuterium density 10-fold compared to palladium alone. The layer also has a very negative free energy, so that the transition metal Pd serves as a source of interface electrons to screen the positive charges of the deuterons from one another [12], thereby facilitating fusion and transmutation. It is thought that fusion may have occurred between deuterons to form helium, 4He2, which then further fuses with the heavier nuclei to give the end product.
Laurence Hecht, editor of 21st Century Science and Technology commented that Iwamra’s work implies a revolution in our understanding of the nucleus, a fundamental breakthrough in science, compared to which, practical applications, even one so necessary as a new supply of cheap, clean energy, is of secondary importance [13].
The most common products of conventional thermonuclear fusion are about 3 to 4 MeV, and that involves an enormous amount of energy input to accelerate apha particles to one-tenth the velocity of light. Iwamura’s transmutation yields 50 to 67 MeV, with the greatest of ease, or very little energy input by comparison.
Allen Widom at Northeastern University Boston and Lewis Larsen of Lattice Energy recently proposed a mechanism that could account for a wide range of fusion and transmutation reactions [7] (for an accessible account read How Cold Fusion Works [2], SiS 36). They suggested that the surface of metallic hydrides fully saturated with protons develop collective electron and proton surface plasma oscillations (plasmons) that enable the electrons to gain sufficient mass to be captured by protons resulting in ultra-low momentum neutrons. In a subsequent paper, they showed how these ultra-low momentum neutrons could be absorbed (captured) by heavier nuclei to produce new elements across the Periodic Table [14]. The expected chemical nuclear abundances resulting from such neutron absorption fit the available low energy transmutation experimental data quite well.
The important feature of such nuclear transmutations is that they do not need special mechanisms to penetrate the high Coulomb barrier, as proposed in other models.
First of all, the experimental distribution in atomic mass number A of the low energy nuclear reaction products measured in laboratory chemical cells are similar to the nuclear abundances found in our local solar system and galaxy. Furthermore, these maxima and minima in abundances resemble those predicted in the ultra-low momentum neutron absorption reaction cross-section (the likelihood of interactions), treating the neutron as a wave. Thus, it raises fundamental questions as to whether the conventional astrophysical account of how the elements are created in our stars and galaxies under thermonuclear conditions is correct.
The prediction based on treating the ultra-low momentum neutron as a wave results in a quasi-periodic curve: the peaks of reaction corresponds to the neutron wave fitting inside the spherical model potential wells of the nuclei, the radius of the well varying with atomic mass.
Data on the yields of transmutation product in an experiment using light water containing Li2SO4 in an electrolytic cell are plotted on the graph (see Figure 2). As can be seen, there is a reasonable correspondence between the experimental points and the predicted peaks and troughs of the neutron cross-section. The magnitude of the transmuted nuclear yields varies from one experimental run to another, but the agreement with the predicted curve remains over all experiments, and regardless of whether the electrode is titanium hydride, palladium hydride or layered Pd-Ni hydride.
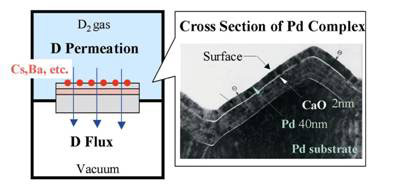
Figure 2. Experimental abundance of elements (filled circles) superimposed on neutron absorption cross-section as a function of atomic mass (continuous line)
When the neutron wavelength within the well reaches resonance with the radius of the well, a peak appears in the scattering strength. If we associate resonant couplings with the ability of the neutron to be virtually trapped in a region near the nucleus, then for intervals of atomic mass numbers around and under the resonant peaks, we could expect to obtain recently discovered neutron ‘halo’ nuclei (nuclei that have a clear separation between a normal core nucleus and a loosely bound low-density ‘halo’ of neutrons outside the core). The spherical potential well model predicts the stable regions for the halo nuclei and thus the peaks in observed nuclear transmutation abundances.
The neutrons yielding the abundances in our local solar system and galaxy have often been previously assumed to arise entirely from thermonuclear processes and supernova explosions in the stars. These assumptions may be suspect in the light of the evidence from low energy nuclear reactions. Widom and Larsen remark: “It appears entirely possible that ultralow momentum neutron absorption may have an important role to play in the nuclear abundances not only in chemical cells but also in our local solar system and galaxy.”
The story of our universe has been created may well have to be rewritten.
Article first published 24/10/07
Comments are now closed for this article