Greening China
Cropland soils are turning acid from the overuse of nitrogen fertilizers, decreasing productivity, polluting the environment, and contributing huge amounts of greenhouse gas emissions; researchers recommend reducing fertilizer use, but have not considered phasing it out altogether by adopting organic agriculture Dr. Mae-Wan Ho
There has been a significant decline in soil pH since the 1980s in China’s major croplands, mainly from the overuse of nitrogen fertilizers. This was revealed in a study carried out by Chinese, UK and US researchers led by Zhang Fu Suo at the China Agricultural University in Beijing [1].
“Serious soil acidification will threaten food security and environmental safety worldwide,” Zhang said [2]. “Our work has shown that soil quality or soil health should be paid more attention in intensive agricultural production systems receiving high nitrogen and other resource inputs.”
The researchers recommend optimal nutrient-management strategies that can significantly reduce nitrogen fertilizer rates without compromising crop yield, but have not considered adopting organic agriculture and phasing out nitrogen fertilizers altogether.
Soils are strongly buffered by inorganic ions, by the weathering of soil mineral, and in the acidic range, by interactions with aluminium and iron, so that its pH remains relatively constant. (pH is a measure of acidity and alkalinity on a scale of 0 to 14; 7 being neutral; it is approximately equal to the negative logarithm (base 10) of the hydrogen ion (H+) concentration.)
Soils become acid very slowly under natural conditions, over hundreds to millions of years. Old soils and soils in high rainfall regions tend to be more acid. Naturally acid soils occupy approximately 30 percent of the world’s ice-free land and are commonly associated with phosphorus deficiency, aluminium toxicity, and reduced biodiversity and productivity.
Chinese agriculture has intensified greatly since the early 1980s on a limited land area with large inputs of chemical fertilizers. Grain production and fertilizer nitrogen consumption reached 502 Mt and 32.6 Mt respectively in 2007, increasing 54 and 191 percent since 1981. High levels of N fertilizer can acidify soils both directly and indirectly, and the rates of N applied in some regions are very high compared with those of North America and Europe. This has degraded soils and environmental quality in the North China Plain and the Taihu Lake region in south China, traditionally famous for its scenic beauty but now infamously putrid and polluted [3, 4].
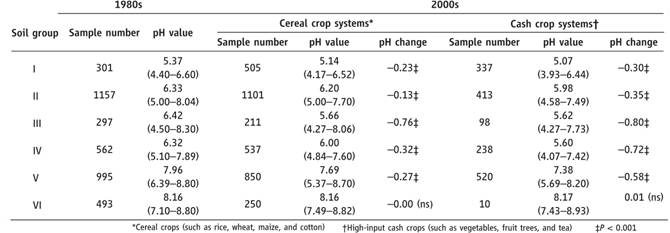
A national soil survey had been conducted during the early 1980s, and pH was determined in all top soils sampled. For comparison, the team collected all published data on top soil pH from 2000 to 2008 and compiled two (unpaired) datasets on the basis of six soil groups according to geography, and two subgroups of cereal crops and cash crops. Both cropping systems receive very high fertilizer inputs compared with other agricultural systems worldwide, especially cash crops like greenhouse vegetables that have expanded rapidly since the 1980s.
The results showed significant drops in pH of 0.13 to 0.8 except in the highest pH soils, which represent only a small percentage of Chinese cultivated soils. In all other soil groups, acidification has been greater in cash crops (pH decreased by 0.3 to 0.8) than cereals (0.13 to 0.76) (see Table 1). As the scale is logarithmic, a pH decrease of 0.3 corresponds to a doubling in hydrogen ion activity.
Soils in group 1 are the most acidic in south China and have acidified further since the 1980s. Athough the net pH decreases for group I soils were small compared to the other groups, the impact may be more pronounced because these soils are approaching acidity at which potentially toxic metals such as aluminium and manganese could be mobilized.
These broad comparative results are corroborated by data from 154 agricultural fields in which strictly paired measurements from the same sites in the 1980s and the 2000s are available. The average decline in pH in these sites is well over 0.5.
Yet more data from 10 long-term monitored field sites in which soil pH was measured regularly over 8 to 25 years also showed decreases in pH ranging from 0.45 to 2.20, only in NPK fertilized plots and not in unfertilized soils, or soils with no crop planted.
In the three major Chinese double-cropping systems – wheat-maize, rice-wheat, and rice-rice – annual N fertilizer application rates are usually above 500 kg N/ha, with nitrogen efficiencies of only 30 to 50 percent. (Nitrogen use efficiency is generally defined as production or carbon fixation per unit nitrogen taken up.) In these systems, ammonium and nitrate N indicate that N loading contributes to increasing 20 to 33 kmol H+/ha/year. Greenhouse vegetable systems, the major cash crops, receive even greater N fertilizer inputs. In Shandong province, N fertilizer rates above 4 000 kg N/ha/y are common, with N use efficiency well below 10 percent. Under this management, about 220 kmol H+/ha/y accumulates. The proton (H+) generation related to N – 20 to 221 kmol/ha/y – in China is extremely high compared with values of 1.4 to 11.5 kmol/ha/y associated with the lower N fertilizer rates in other countries.
Plant uptake of base cations (positively charged metal ions), which are then removed as harvests from fields, also leads to soil acidification because it leaves behind excess anions (negatively charged ions) that are balanced by an equivalent amount of H+ released to the soil. Currently, about 25 tonnes of dry biomass are harvested annually in the three double-cropping systems, resulting in an estimated release of 15 to 20 kmol H+ /ha/y that compensates for the base cations removed. In the greenhouse vegetable systems, the importance of base cations uptake varies greatly with plant species and yield but overall appears similar to the cereal systems.
Thus, the total H+ added to the soil due to nitrogen fertilizers and base cation removal is 30 to 50 kmol H+/ha/y for cereal systems, and 230 kmol/ha/y for greenhouse vegetable systems. In comparison, acid deposition due to acid rain is negligible, at 0.4 to 2.0 kmol/ha/y.
Each kg of applied ammonium-N leached as nitrate-N requires 7.2 kg of CaCO3 to neutralize, which is very expensive.
Soil acidification occurs not just in China, but wherever and whenever intensive chemical fertilization agriculture is practiced in response to pressures to produce more food, and recently, bioenergy crops for biofuels [5] Biofuels: Biodevastation, Hunger & False Carbon Credits (SiS 33), which means even less land for growing food in developing countries.
The overuse of chemical fertilizers is a major source of environmental pollution from agriculture, which China’s recent national pollution census identified to be greater than industry [6] China's Pollution Census Triggers Green Five-Year Plan (SiS 46)
Collaborating scientists Keith Goulding and David Powlson of UK’s Rothamsted Research Institute highlight another important aspect of chemical fertilization [7]: 'The impact of N fertilizer over-use on greenhouse gas emissions is often overlooked. It arises through the carbon dioxide emitted when manufacturing fertilizer, and nitrous oxide, a powerful greenhouse gas, emitted when N fertilizer is applied to soil. Our work with Chinese collaborators shows that reductions in N use of 30 percent and, in some cases, much more are possible without any threat to China's food security, and would make a significant contribution to reducing total greenhouse gas emissions from China. Avoiding N fertilizer over-use is a “multiple win”: farmers save money, there is less water pollution, smaller greenhouse gas emissions, and a smaller acidification burden on soil and water.”
The real solution is to phase out chemical fertilizers altogether in favour of organic fertilizers [8] (see Sustainable Agriculture, Green Energies and the Circular Economy, SiS 46).
Article first published 30/03/10
Comments are now closed for this article
There are 7 comments on this article.
caglar Comment left 10th May 2010 05:05:16
I am a farmer and growing vegetables to sell. For more efficency i use fertilizers but while using them it is important to
keep it healthy because some fertilizers contain corruptive elements so i try to read everything about fertilizers and try
to keep my product healthy. I am grateful for those who gives information about fertilizers and anyone who
uses fertliziers should read about it, i also found another good guide which should be read too i think;
http://agricultureguide.org/
Jeffrey Michel Comment left 30th March 2010 22:10:40
Excessive coal usage is probably compounding this problem. Here in eastern Germany, the use of high-sulphur lignite had measurable effects on plant growth. Some streams became so acidic that trout could no longer spawn. Automobile emissions, arising largely in western Germany and falling on local cultivated fields were calculated to be equivalent to the amount of artificial fertlizer that German farmers used in the 1930's.
Paulo Ramos Comment left 30th March 2010 22:10:22
It is a very important information which doesn´t get most farmers that are also victims of bad technical assistance. The promisses of production!
I would like to have more detailed informations about the processes by wich Nitrogen based fertilizers or wild fire retardants turn soils acid and the implications for the atmosferic concentrations of NO2 for example.
Mae-Wan Ho Comment left 1st April 2010 01:01:02
The most important acid forming reactions for N fertilizers is microbial oxidation of ammonium compounds and urea to nitrate, especially when it N fertilizers are added in excess of what the plants can assimilate.
NH3 + 2O2 → H+ + NO3- + H2O
The most important acid forming reactions for N fertilizers is microbial oxidation of ammonium compounds and urea to nitrate, especially when it N fertilizers are added in excess of what the plants can assimilate.
NH3 + 2O2 → H+ + NO3- + H2O
The most important acid forming reactions for N fertilizers is microbial oxidation of ammonium compounds and urea to nitrate, especially when N fertilizers are added in excess of what the plants can assimilate.
NH3 + 2O2 → H+ + NO3- + H2O
CO (NH2) + 4O2 → 2H+ + 2NO3- + H2O + CO2
It is the protons (H+) generated in the nitrification reaction that causes acidity.
When plants assimilate nitrate, i.e., when they take up the nitrate and react it with an organic compound (R-OH) they consume protons, as follows:
R-OH + NO3- + H+ → R- NH2 + O2
So if nitrogen is in balance, there should be no acidification of the soil.
Rory Short Comment left 1st April 2010 01:01:47
Our arrogance as a species is appalling and will no doubt lead to our disappearance. A little knowledge is a dangerous thing and the current situation with regard to the processes of life is that we still have insufficient knowledge to be confident of the outcomes when meddling with them.
Santhanam R. Comment left 30th March 2010 22:10:55
Well one got the impression that China was the role model to follow especially after the self declaration that China will become fully organic in a few years?
The earthworm gut can make soils richer, reduce acidity. Other is to use stabilised bio solids from segregated MSW and apply them to farm lands instead of dumping in land fills , emitting GHG.
Two technologies from India can help achieve these: Biosanitizer and Vrikshayurveda a Vedic sciences based proprietary knowledge based farming.
lewse bitch 101 Comment left 14th March 2011 20:08:24
look there are no effects displayed here and effects are just as important as the problem