Chardon LL T 25 Maize Hearing
Evidence with special emphasis on the use of glufosinate ammonium (Phosphinothricin)
Professor Malcolm Hooper Ph.D, B.Pharm., MRIC, C Chem
Summary Of Curriculum Vitae
I obtained the following degrees from the Faculty of Medicine, University of London, B.Pharm. (1956), Ph.D.(1959). By election I received C.Chem. MRIC in 1963.
1959: Appointed Lecturer in Pharmaceutical and Medicinal Chemistry, School of Pharmacy, Sunderland Technical College.
1963: Appointed Senior Lecturer in Pharmaceutical and Medicinal Chemistry, School of Pharmacy, Sunderland Technical College.
October 1969: Appointed Reader in Pharmaceutical and Medicinal Chemistry, School of Pharmacy, Sunderland Polytechnic.
March 1982: Appointed Professor in Medicinal Chemistry, Faculty of Pharmaceutical Sciences, Sunderland Polytechnic.
August 1992: Retired as Professor of Medicinal Chemistry.
September 1993: Appointed Emeritus Professor of the University of Sunderland.
Throughout this period I taught students of pharmacy, pharmacology, and pharmaceutical and chemical analysis at honours degree level.
I have directed research at masters and particularly doctoral level with a special emphasis on the development of drugs for the treatment of tropical diseases.
I have published in peer reviewed journals in the field of medicinal chemistry together with major reviews on the Chemotherapy of Leprosy, the Chemistry of Isatogens. I have edited one book on the Chemotherapy of Tropical Diseases.
I have acted as a referee for a number of important journals and served on one editorial board.
I have served on Committees of the Council for National Academic Awards, CNAA, World Health Organisation, Science and Engineering Research Council.
I am a member of a number of learned societies including the Royal Chemical Society, the British Pharmacological Society, the Society for Drug Research, SDR, (now renamed as the Society for Medicines Research). For many years I was on the committee of the SDR and served as Chairman for 2 years. This involved the planning and organising of major national and international conferences.
Since 1997 I have been involved with Gulf War Veterans and the question of Gulf War Syndrome. I was appointed Chief Scientific Advisor to the Gulf War Veterans Association and accepted by the Ministry of Defence as their nominee on the Independent Panel established to consider the possible interactions between Vaccines and NAPS tablets and the Depleted Uranium Oversight Board. I also serve on the Gulf Support Group convened at the Royal British Legion which brings together scientists, politicians, welfare agencies and Gulf War Veterans. My involvement with the Gulf Veterans brought me into contact with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, CFS/ME, and related disorders. And issues surrounding the use of pesticides and herbicides.
My concerns about health and the environment has drawn me into an examination of the development and testing of GM crops and foodstuffs with a special interest in the consequences of the, possibly widespread, use of herbicides and the consequences of novel gene constructs, which include antibiotic resistance genes.
Medicinal Chemistry deals with the science associated with the design, development and testing of new chemicals for the treatment of disease, pest and weed control.
Introduction
In the major part of my evidence I shall deal with important questions associated with-
- The presence of the PAT gene that confers resistance to glufosinate ammonium.
- The obligatory use of glufosinate ammonium as a broad-spectrum herbicide as part of the strategy for growing GM crops, especially Chardon LL T 25 that is the subject of this hearing.
- The relationships between glufosinate and the important biological molecules glutamine and glutamate.
- I have not considered any significant genetic aspects of Chardon LL as these have been covered experts in this field ( Ho, 2002; FoE, 2000a)
I shall deal briefly with some aspects of other evidence presented to the hearing in a closing paragraph.
The PAT (phosphorthricin acetyl transferase) Gene
- This gene, which is part of the five-gene construct, confers resistance to glufosinate ammonium that is used as a broad-spectrum herbicide with the Chardon LL T 25 maize crop.
- The gene-protein is a completely novel protein/enzyme and has been found only in a mutant bacterium. This has important implications for food safety (FoE, 2001a,b). It provides the GM plant with the ability to metabolically inactivate, by N-acetylation, the glufosinate molecule thereby rendering it ineffective as a herbicide.
- However, the N-acetylated glufosinate molecule is not further destroyed but is stored in the plant as the inactive N-acetylphosphinothricin.
- It is very important to recognise that this is a feature only of the GM crop. Although the only reported studies, I have been able to find, concern only, plant cell cultures of sugarbeet, carrot, purple foxglove and thorn apple (Muller et al. 2001), or canola (rape) (Beriault et al. 1999) it is reasonable to assume that this also occurs in the Chardon LL maize.
- If a similar study of Chardon LL has not been done it represents a significant failure by the Company and must be done forthwith. If the data is only in Company files then it must be released immediately.
- N-acetylphosphinothricin is not found in the natural crop and represents a very significant difference in bio-equivalence between the transgenic and non-transgenic crops- see also below.
- Critically, data supplied by AgrEvo, now Aventis, demonstrates that micro-organisms, in the digestive tract of warm-blooded animals, can remove the inactivating acetyl group and regenerate the active and toxic phosphinothricin (glufosinate) (Jewell and Buffin, 2001).
- This is clearly very serious and means that ALL transgenic crops, incorporating the PAT gene, and materials obtained from them, eg. oils and manufactured products, must be fully evaluated for the presence of this metabolite of phosphinothricin.
In my judgement, all field trials must be halted immediately until critical questions about the metabolism, storage and reconversion of the N-acetylphosphinothricin have been fully answered for all PAT gene containing products.
Glufosinate Ammonium (Phosphinothricin)
- This is a novel metabolite isolated from soil bacteria, various Streptomyces spp., (Merck Index, 1996).
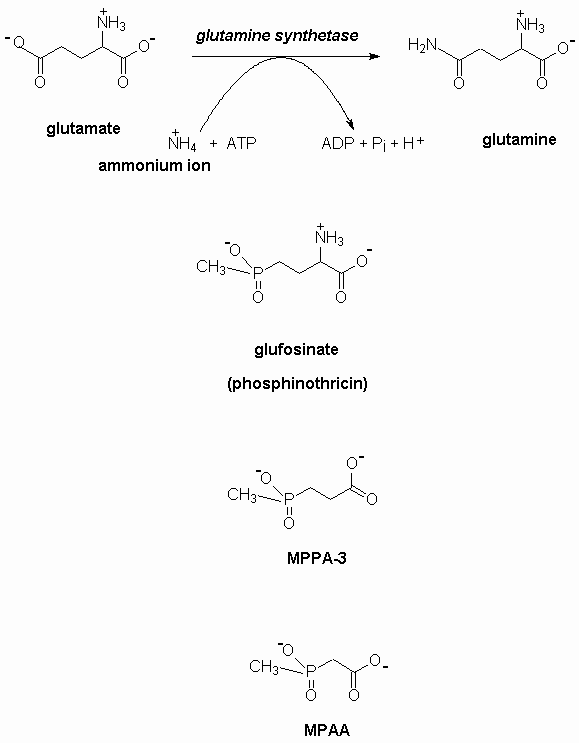
- Its acts by inhibiting the enzyme, glutamine synthetase, which converts the essential amino acid, glutamic acid to glutamine. There are clear molecular similarities between phosphinothricin, glutamate and glutamine- see Appendix 1 for more details. The enzyme, glutamine synthetase is ubiquitous and occurs in plants, insects and animals including humans and farm animals.
- The net result of the action of glufosinate is that ammonia and glutamate accumulate whilst glutamine is limited. It is the accumulation of ammonia that is the lethal action in plants. However the action is limited by the distribution of glufosinate in plants where it is restricted to the leaves and the stem which come into direct contact with the sprayed material (Jewell and Buffin, 2001). Deep rooted plants and stolons are not destroyed. Glufosinate is not a universal herbicide.
- In mammals the consequences of inhibition of glutamine synthetase are more associated with the increased levels of glutamate, and decreased levels of glutamine, see below. Circulating ammonia is removed in the liver by, an alternative mechanism, the urea cycle. However, the brain is highly sensitive to the toxic effects of ammonia and the removal of excess ammonia depends on its incorporation into glutamine (Zonehome, 2002).
Toxic Effects of Glufosinate
- In Humans
- Most of this information comes from acute overdoses taken accidentally or as a means of committing suicide. Since 1989 many such cases have been reported from Japan (Watanabe and Sano, 1998).
- Such poisonings involve not only glufosinate, the pharmacologically active compound, but also other components of the formulated product such as surfactants which may be present in very large amounts and may not have been tested for their toxicological properties. From studies in rats the surfactant was found to have cardiostimulatory and vasodilative effects at low doses but cardiosuppressive effects at high doses. These changes were consistent with the cardiovascular changes found in clinical situations (Koyama, Koyama and Goto, 1997).
- Neurotoxicity is characteristic of glufosinate poisoning although the mechanism is not clear. Generalised convulsions, mental disturbances and short-term memory loss have all been observed (Watanabe and Sano, 1998; Tanaka et al., 1998).
- Impaired respiration, gastrointestinal effects and haematological changes were also observed.
- Analysis of previously published cases appears to indicate that the toxic effects arise from both the active ingredient and the surfactant (sodium polyoxyethylene alkylether sulphate, AES) in the formulation (Koyama, Koyama and Goto, 1997).
- A toxico-kinetic analysis in a patient with acute glufosinate poisoning found that there were various neurological symptoms, disturbances of consciousness, convulsions and apnea which appeared after an asymptomatic interval of several hours. Four and a half hours after ingesting around 300 ml of a 20% solution of glufosinate the patient showed speech ataxia and systemic tremor and was intubated prior to serious respiratory failure. Five days later he was discharged without any sequalae. Changes in urinary excretion were monitored and from the data it was concluded that excretion could be modelled by a two-compartment model that showed glufosinate was eliminated from the first compartment with a T1/2 of 1.84 hours and the second compartment with a T1/2 of 9.59 hours (Hirose et al. 1999). A similar study in rabbits proposed a similar two-compartment model although renal clearance values were much higher -a common feature with small animals. Glufosinate appears to be handled in a manner similar to that for theophylline, caffeine and pentobarbital (Koyama et al. 1998).
- In vitro studies of glufosinate binding to human serum albumin concluded that glufosinate was rapidly eliminated by the renal route but such studies cannot be expected to mimic the much more complex situation which involves whole blood in the whole animal; many other binding molecules and cells are present (Hori et al, 2001).
- A transient diabetes insipidus was observed in one case together with disturbed consciousness, convulsions and apnea (Takahashi et al. 2000).
- A case-referent study of 261 matched pairs examined the possible relationship between pesticide exposures and congenital malformations. It found an odds ration of 2.45(95% CI 0.78-7.70) for glufosinate exposure. The wide confidence intervals show the study did not reach statistical significance and that it needs to be extended. Nevertheless, it is reasonable to conclude that "there is a possible risk of congenital malformations for paternal exposure to …….. Glufosinate". This is a deeply worrying finding and must be followed up as a matter of urgency. No further trials of glufosinate associated transgenic crops can be allowed until this issue is resolved (Garcia et al., 1998).
- The mammalian metabolite of glufosinate, 3-methylphosphinylpropionic acid (MPPA-3) is also a known neurotoxin and needs to be considered in assessing the human toxicology of glufosinate (US EPA, 1988).
- In my judgement, a further metabolite, methylphosphinlyacetic acid, MPAA, might also have toxic effects. I could find no studies on this important topic.
- It is important to note that oral administration of glufosinate has major effects on the brain. This indicates that the blood-brain barrier, which prevents access of many compounds to the brain, is readily crossed by glufosinate.
- Many of the toxic effects of glufosinate are consistent with the known pharmacology and physiology of glutamine and glutamate- see below. A recently drug introduced to treat epilepsy, lamotrigine, acts by blocking glutamate receptors in the brain.
- In Laboratory Animals
- Rats and mice are the most common laboratory animals used to study the pharmacology and toxicology of novel compounds being developed for human use. Although species variations do occur it is possible to extrapolate data from studies with these small animals to man. The drug industry is adept at making such extrapolations. Most drugs have come into common usage following the identification of important pharmacological properties in these and other small animals. Similarly toxicological studies routinely involve small animal work. On ethical grounds the use of cell culture systems is being rapidly extended to reduce the volume of work with small laboratory animals.
- Glufosinate Ammonium induces convulsions in mice via N-methyl-D-aspartate, NMDA, receptors. This is consistent with the observed response in humans when glufosinate is ingested, see above, and the induced aggressiveness, wet dog shakes and limbic seizures in rats (Matsumura et al. 2001). The drug was administered by intraperitoneal injection showing again that peripherally administered glufosinate readily enters the brain. Glufosinate has been shown to bind to L-glutamate binding sites in rat brains. This study clearly demonstrates that the main mechanism of action of glufosinate is by its agonist effect at glutamate receptors- see below.
- Stimulation of the NMDA receptors has been shown to stimulate nitric oxide production in vivo (Nakaki et al. 2000). Nitric oxide has many roles in the body and affects blood flow, the immune response and serves as a neurotransmitter. It is a free radical and in excess would exert oxidative stress in the tissues involved.
- Using whole embryo culture techniques glufosinate ammonium was found to induce apoptosis (programmed cell death) in neuroepithelial cells in the developing mouse embryo. This teratogenic action is extremely disturbing and would undoubtedly result in a drug being withdrawn from the further development (Watanabe, 1997).
- Maternal exposure to a single exposure of glufosinate during the time of neurogenesis in the hippocampus lead to a decrease in the wet dog shakes response to kainic acid. This indicates that a different set of glutamate receptors were affected leading to functional abnormalities in the brains of the offspring (Fujii 1997). Yet again such a property would lead to withdrawal of a drug from development. I have no where found any response by Aventis or its precursor company to this damning evidence published some 5 years ago.
- The mortality in mice to high doses of glufosinate was significantly increased depending on which stage of the circadian cycle the compound was given. Mortality was greatest when administration took place in the light phase. This is disturbing since the glufosinate will, generally, be applied in daylight (Yoshiyama et al., 1995).
- Towards Insects
- In a complex process L-glutamate in the moulting fluid of the Brazilian skipper butterfly in converted to glutamine by the enzyme glutamine synthetase. The toxic effects of glufosinate towards the pupae of this butterfly are caused by inhibition of this process (Yarema et al. 2000). Since this process appears to be general for all Lepidoptera it is likely that glufosinate will damage related insects in a similar manner.
- Glufosinate is also toxic to the fifth instar of the skipper butterfly, causing cessation of feeding, neurotoxic as indicated by proleg tremors, body convulsions and complete paralysis that results in death. The toxic dose was below that normally delivered during spraying. These effects were shown to be due to glutamine depletion in the caterpillar (Kutlesa and Caveney, 2001).
- Some developmental forms of insects that perform a valuable predatory role against plant pests, such as Tetranychus urticae, are adversely affected by glufosinate which however is more toxic towards the developmental stages of the pest insect. It seems that there is a similar mechanism of action in both the predatory and pest insects. Further study of this complex web of relationships is needed (Ahn et al., 2001).
- Glufosinate is highly toxic to some beneficial insects such as phytoselids and bagworm moths (Jewell and Biffin, 2001).
- Other Life Forms
- Glufosinate is toxic towards the larvae of clams and oysters, daphnia and some freshwater fish; the rainbow trout is the most sensitive species of those tested (Jewell and Biffin, 2001). Run off from sprayed areas into streams and lakes could be damaging to some fisheries.
- Inhibition of some beneficial soil bacteria and fungi by glufosinate is known. Some 37% of the organisms tested were sensitive to and adversely affected by glufosinate. In boreal forest soils 20% of the fungi and 40% of the bacteria were sensitive to glufosinate. Nitrogen fixing soil bacteria and rhizobial nodulation rates were reduced, in some cases drastically, in tests in sterile soils. Although soils are not sterile the possible impact of glufosinate on nitrogen fixation rates is worrying and needs much more detailed investigation (Jewell and Biffin, 2001).
- Cellulose decomposition is also inhibited by glufosinate even at low concentrations. Burial of cellulose substrates in soils sprayed with 150 ppm of glufosinate resulted in a 78% reduction in cellulose decomposition rates (Jewell and Biffin, 2001).
- Some plant pathogens were found to be highly resistant to glufosinate but, in contrast, organisms antagonistic to these pathogens were seriously and adversely affected. The loss of these antagonistic organisms could have far reaching effects (Jewell and Biffin, 2001).
- Glufosinate which is a broad spectrum herbicide would be expected to damage all plants that it came in contact with thereby reducing the biodiversity of the environment in which it was used.
- The knock-on effect of loss of insects and plants would affect bird and small animal life as well as agriculture.
Glufosinate Residues (from Jewell and Biffin)
- In Food
- Glufosinate ammonium as well as being sprayed on to crops to combat weed growth is also used as a pre-harvest desiccant. MAFF has found that consumers are most likely to be exposed to residues in potatoes, dried and processed peas, and liver and kidney from animals fed contaminated cereal straw.
- Spraying with glufosinate in apple orchards is at 540 ppm (Ahn et al., 2001)
- The WHO/FAO recommended ADI (Allowed Daily Intake) is 0.02mg/kg of body weight, ie. 20 ppb (parts per billion). Equivalent to a total dose of 1.4 mg for a 70 kg person.
- The highest likely residue levels were considered to be
3 mg/kg = 3 ppm (parts per million) dried peas.
1 mg/kg wheat grain.
0.5 mg/kg oilseed rape seeds.
Edible parts of spinach, radishes, wheat and carrots were found to contain residues some 120 days after the application of glufosinate.
In potatoes 0.1 mg/kg were found but the toxic metabolite MPPA-3 was found at 0.07 mg/kg (single application) and 2.4 mg/kg (double application- this is more common) some 77 days after treatment.
Flour contained 10-100 % of the wheat residues
Bran contained 10 -600 % of wheat residues.
In animal feed
50 mg/kg in barley straw and pea stalks.
20 mg/kg in wheat straw and field bean stalks.
- Drinking Water
- Although Aventis claim that glufosinate is unlikely to leach into drinking water supplies it is classed as a persistent and mobile contaminant by the USA Environmental Protection Agency. The company's claims are spurious.
Despite the growing awareness of potent pharmacological compounds finding their way into drinking water only limited testing is done by the Environment Agency; glufosinate is not included in the present testing regimen.
- Persistence in Soil and Water
- This is very variable depending on the nature of the soil and its organic content. The half-life varies from 3 to 42 days in some studies but reached up to 70 days in others. Soil coverage was also important as was temperature and soil moisture content. Glufosinate was most persistent in sandy soils overlying aquifers where its transport through soil was "essentially unretarded" (Richelle et al., 1995).
- The metabolite, MPPA-3, has been found to be more persistent and mobile than glufosinate and in soil column leaching experiments was leached some 20 times faster than glufosinate (Jewell and Biffin, 2001).
Glutamine
This amino acid is important for homeostatic functions, body fluid and pH balance, body temperature, and heart rate, and the optimum functioning of a number of body systems particularly the brain, immune system and the gut. It is best described as a "conditionally essential amino acid".
- It is the most abundant free amino acid in the human body.
- Constitutes 50-60% of the total free amino acid pool in skeletal muscle.
- Constitutes 20% of the plasma amino acid pool.
- Readily crosses the blood brain barrier and enters the brain which has 10 to 15 times the concentration in the blood.
- Modulates key neurotransmitter molecules in the brain, the excitatory glutamate and inhibitory g-aminobutyrate, GABA.
- Is the major fuel for cells
- In the gut which utilises 40% of the body's glutamine.
- Brain
- Immune cells
- Kidneys
- Liver
- Has a major role in assisting detoxification processes in these and other organs.
- In the immune system it raises circulating lymphocyte and macrophage levels increasing resistance to infection
- Plays an important role in the regulation of glucose metabolism- increasing glucose production and muscle glycogen storage.
- Plays a key role in nucleic acid synthesis.
- Restores protein in skeletal muscle by anabolic and anti-catabolic mechanisms.
- Protects against stress whether derived from burns and trauma, illness or overtraining in athletes.
- Is produced in the body by glutamine synthetase the primary site being skeletal muscle with the liver, lungs and brain being secondary sites.
Major references Horleys, 2001, Occhipinti, 2001, IoM 1999.
It is difficult to conceive of a more potentially damaging target, for compounds destructive to human health and well-being, than the glutamate-glutamine and glutamine synthetase system. Any disruption of this finely balanced and essential system by compounds such as glufosinate will have far-reaching and long-term consequences.
Because of the ubiquitous nature of this system, which is highly conserved throughout nature, almost every kind of living organism will be open to extensive damage from compounds like glufosinate.
Glutamate and its Receptors
Information about glutamate and its receptors has been available in all pharmacology,
biochemistry, and medicinal chemistry books over many years (see for example, Wingard et al. 1991, Stryer 1988, Krogsgaard-Larsen and Bundgaard 1991).
Glutamate is
- The premier excitatory neurotransmitter in the brain.
- A key compound in the development of the foetal and neonatal brain.
- Important in memory and learning in the adult brain.
- An initiator of programmed cell death, apoptosis, in brain cells.
- Part of an essential balance between excitatory and inhibitory amino acids in the brain.
- A key component of the energy producing cycle, the Kreb's cycle, in all mammalian cells.
- Glutamate is also an excitotoxic neurotransmitter in the enteric nervous system where it can induce apoptosis and cell death. This could result in damage to the gut and loss of structural and functional integrity (Gershon, 1999). The broiler chicken study could be indicative of such damage since the weight gain with T 25 maize falls off in the final stages of the study and death rates are increased (Leeson, 1996; Howard, 2000, FoE, 2001a).
Disruption of the levels of this essential amino acid will have extensive and long-term effects in many living organisms.
Associated Significant Issues
- Other submissions to this Hearing have shown that the scientific studies supporting the claims of the manufacturers supporting the use of glufosinate and transgenic crops is
- Often badly conceived and inadequately assessed
- Does not provide substantive evidence for the claims being made
- Is surrogate science that avoids the crucial experiments that are demanded for the proper understanding of the impact of these new technologies (Howard, 2000; FoE, 2001a,b,c).
- The treatment of some major scientists in the field of GM crops has been outrageous and quite contrary to the spirit of scientific search for truth and understanding (Pusztai, 2002).
- There is now clear evidence of attempts to mislead the public and discredit good science by major companies involved in the development and sales of GM maize (Monbiot, 2002).
- The whole political process, in Europe and the United Kingdom has been subverted in the way Chardon LL T 25 has been handled and key evidence ignored (FoE, 2001a,d). T25 maize has been introduced into the food chain illegally (FoE, 2000). Deception and deceit appear to be the order of the day.
- There is no consideration of the impact of other genetic material which is part of the gene-construct necessary to introduce the glufosinate resistance gene. In particular the use of an ampicillin resistant gene derived from E coli. This gene will ensure the even greater and faster spread of penicillin resistance-now a major clinical problem. The wilful and calculated spread of such resistance is irresponsible and foolhardy. The use of such genes has been strongly advised against by various key groups, including the British Medical Association (FoE, 2001).
- Although my evidence is limited to key questions surrounding Chardon LL T25 maize the same criticisms can be levelled against any crops that use the glufosinate resistance gene construct, sugarbeet and rape, or where glufosinate is used as a desiccant.
- Similar transgenic systems using glyphosate as the broad spectrum herbicide are open to the same kinds of criticism.
Conclusions
Glufosinate is not only a broad spectrum herbicide but also a highly selective and active compound that undoubtedly adversely affects many other organisms because of the ubiquity of the target enzyme system.
In particular glufosinate seriously damages the same system in humans with consequences for foetal, child, and adult health.
It is difficult to imagine a compound with greater potential threat to human and environmental health than glufosinate.
Glufosinate, if it were a drug candidate, would have been withdrawn from development years ago on account of its teratogenic and neurotoxic properties.
Many more well-designed scientific studies are required to investigate the known as well as the potential effects of glufosinate on humans and the wider environment.
Scientific study has been prostituted to support the claims of manufacturers and interests of global organisations intent upon dominating world food production. This has lead to poor science that is partial, generally ill-designed, and does not address the key questions.
Very disturbing are attempts to discredit good science, that does not provide conclusions that are acceptable to company claims, and threatens their product(s).
Equally disturbing are attempts to manipulate scientific and other literature in order to mislead the public. The truth is not being told.
The widespread testing of GM maize and other crops in field trials is premature and irresponsible in the extreme. Almost every assumption underlying their testing has been shown to be wrong.
The use of the UK countryside as an open laboratory for these studies is unacceptable at the present time and must end immediately. It should only resume when sound independent scientific studies have been fully completed and with the agreement of the local communities involved.
The introduction of GM foods into the human and animal food chains at the present time is utterly irresponsible and foolhardy in the light of our present scientific knowledge of these crops and their effects on human health and the environment.
All such crops should be withdrawn immediately to allow essential and independent scientific studies to be carried out. In particular, the mixing of produce from GM and non-GM crops must cease. Such mixing, for the purpose of sales and to hoodwink the public, must stop. The support of the USA and UK Governments for such practices is reprehensible and dangerous.
Article first published 02/07/02
References
- Ahn YJ, KimYJ, Yoo JK. Toxicity of the herbicide glufosinate-ammonium to predatory insects and mites of Tetranychus urticae (Acari:Tetranychidae) under laboratory conditions. J Economic Entomology 2001, 94, 157-61.
- Beriault J, Horsman G, Devine M. Phloem transport of D,L-glufosinate and acetyl-L-glufosinate ammonium in glufosinate-resistant and susceptible brassica napus. Plant Physiology 1999, 121, 619-628. Quoted in Cummins J. (Norfolk Genetic information Network) Ignored side effects of glufosinate herbicide.
- http://members.tripod.com/~ngin/160202a.htm.
- FoE 2000, Illegal GM Food available at http:/www.foe.co.uk/pubsinfo/briefings/html/20001106170722.html
- FoE 2001 Antibiotic Resistance Genes in GM Foods available at http:/www.foe.co.uk/resource/briefing/antibiotic_resistant_genes.html
- FoE 2001a, Sound Science? The evidence against Aventis' GM maize see www.foe.co.uk
- FoE, 2001b, Environmental Audit Committee Briefing available at http:/www.foe.co.uk/resource/briefing/environmental_audit_committee.html
- FoE, 2001c, GMOs: The Case for a Moratorium available at http:/www.foe.co.uk/resource/briefing/gmos_case_for_moratorium.html
- FoE, 2001d, GMOs: Scientific reasons for concerns in The Case for a Moratorium available at http:/www.foe.co.uk/resource/briefing/gmos_case_for_moratorium.html
- Fujii T. Transgenerational effects of maternal exposure to chemicals on the functional development of the brain in the offspring. Cancer Causes and Control: CCC 1997, 8, 524-28.
- Garcia AM, Benavides FG, Fletcher T, Orts E. Paternal exposure to pesticides and congenital malformations. Scandinavian Journal of Work , Environment and Health 1998, 24, 473-480.
- Gershon MD. The Second Brain. Harper Perennial, New York, 1999.
- Hirose Y, Kobayashi M, Koyama K, Kohda Y, Tanaka T, Honda H, Hori Y, Yoshida K. A toxicokinetic analysis in a patient with acute glufosinate poisoning. Human and Experimental Toxicology 1999, 18, 305-8.
- Ho M-W. Promoting Critical Public Understanding of Science and Enhancing the GM Debate. Institute of Science in Society available at www.i-sis.org Many other key publications are available at this site.
- Hori Y, Koyama K, Fujisawa M, Nakajima M, Shimada K, Hirose Y, Kohda Y, Akuzawa H. Protein binding of glufosinate and factors affecting it revealed by an equilibrium dialysis technique. J Analytical Toxicology 2001, 25, 439-442.
- Horleys. What is Glutamine. http://www.horleys.com/html/article_glutamine_body.html Download 13th May 2002. This report includes many useful references.
- Howard CV. Analysis of key documents relevant to the safety of Chardon LL for animal feed purposes. Chardon LL Hearing, October 2000.
- Institute of Medicine. The Role of protein and Amino Acids in Sustaining and Enhancing Performance. Committee on Military Nutrition Research. National Academy Press, Washington DC 1999. Available at http://www.nap.edu/openbook/0309063469/html/R1.html
- Jewell T, Buffin D. Health and environmental impacts of glufosinate ammonium. Pesticides Action Network, 2001.
- Koyama K, Kohda Y, Hisashi H, Koyama Ky, Goto K. Toxicokinetics of glufosinate, an herbicide structurally analogous to glutamic acid, that causes severe CNS disorders in human acute oral poisoning. Toxicology Letters 1998, 95 (Suppl 1), 140.
- Koyama K, Koyama K, Goto K. Cardiovascular effects of a herbicide containing glufosinate and a surfactant: in vitro and in vivo analyses in rats. Toxicology and Applied Pharmacology 1997, 145, 409-414.
- Krogsgaard P and Bundgaard H (editors). A Textbook of Drug Design and Development, Harwood Academic Publishers, Chur, 1991.
- Kutlesa NJ, Caveney S. Insecticidal activity of glufosinate through glutamine depletion in a caterpillar. Pest Management Science 2001, 57, 25-32.
- Leeson S. The Effect of Glufosinate-Resistant Corn on the Growth of Male Broiler Chickens. Report No A56379, July 1996.
- Matsumura N, Takeuchi T, Hishikawa K, Fujii T, Nakaki T. Glufosinate ammonium induces convulsions through N-methyl-D-aspartate receptors in mice. Neuroscience Letters 2001, 304, 123-5.
- Merck Index 12 edition Entry 7495. Merck and Co Inc. Whitehouse Station NJ, 1996.
- Monbiot G. Guardian !5th May 2002.
- Muller BP, Zumdick A, Schuphan I, Schmidt B. metabolism of the herbicide glufosinate-ammonium in plant cell cultures of transgenic (rhizomania-resistant) and non-transgenic sugarbeet (Beta vulgaris), carrot (Daucus carota), purple foxglove (Digitalis purpurea) and thorn apple (Datura stramonium). Pest management Science 2001, 57, 46-56.
- Nakaki T, Mishima A, Suzuki E, Shintani F, Fujii T. Glufosinate ammonium stimulates nitric oxide production through N-methyl-D-aspartate receptors in rat cerebellum. Neuroscience Letters 2000, 290, 209-212.
- Occhipinti MJ. Learn how to maximise your physical and psychological potential with the "wonder nutrient" L-Glutamine. http://www.afpafitness.com/Glutamin.htm Download 13th May 2002. Despite its title this report has some useful references.
- Pusztai A. GM Food Safety: Scientific and Institutional Issues. Science as Culture 2002, 11, 69-92.
- Richelle M A-K, Butler BJ, Reichert B. Fate of the herbicide glufosinate-ammonium in the sandy low-organic-carbon aquifer at CFB Borden, Ontario Canada. J Contaminant Hydrology 1995, 18, 161-179.
- Stryer L. Biochemistry, 3rd Edition, WH Freeman, New York, 1988.
- Takahashi H, Toya T, Matsumiya N, Koyama K. A case of transient diabetes insipidus associated with poisoning by a herbicide containing glufosinate. J Toxicology. Clinical Toxicology 2000, 38, 153-6.
- Tanaka J, Yamashita M, Yamashita M, Matsuo H, Yamamoto T. Two cases of glufosinate poisoning with late onset convulsions. Veterinary and Human Toxicology 1998, 40, 219-222.
- US EPA (United States Environmental Protection Agency) 1988. Quoted in Jewell and Biffin.
- Watanabe T, Sano T. Neurological effects of glufosinate poisoning with a brief review. Human and Experimental Toxicology 1998, 17, 35-9.
- Watanabe T. Apoptosis induced by glufosinate ammonium in the neuroepithelium of developing mouse embryos in culture. Neuroscience Letters 1997, 222, 17-20.
- Wingard LB, Brody MB, Larner J, Schwartz A. (editors) Human Pharmacology Molecular to Clinical. Wolfe Publishing, London, 1991.
- Yarema C, McLean H, Caveney S. L-Glutamate retrieved with the moulting fluid is processed by a glutamine synthetase in the pupal midgut of Calpodes ethlius. J Insect Physiol. 2000, 46, 1497-1507.
- Yoshiyama Y, Kobayashi T, Kondo R, Tomonaga F, Ohwada T. Chronotoxicity of glufosinate ammonium in mice. Veterinary and Human Toxicology 1995, 37, 22-3.
- Zonehome Protein and Nitrogen Homeostasis http://www.zonehome.com/met/metprotnit.htm
Appendix
structural relationships between glutamate, glutamine and glufosinate
Comments are now closed for this article
