Closed loop zero-waste chemical processing of waste biomass from agriculture is the future of green chemistry, says Prof. James Clark of York University, UK. Dr. Mae-Wan Ho

Green chemistry is thriving in the UK, as Professor James Clark will tell you. He heads the Green Chemistry Centre of Excellence, York University, and is a founding director of the UK-based Green Chemistry Network. True to the “closed loop” approach that has been making headway in industry and mainstream politics [1] (Closed Loop, Cradle to Cradle, Circular Economy & the New Naturephilia, SiS 49), Clark is especially keen on using waste as a feedstock for ‘biorefineries’ [2, 3].
A typical biorefinery operating on the closed loop principle sequentially extracts and processes waste biomass feedstock to obtain a range of valuable plant products, ending with fuels of various kinds, and the wastes are further recycled for processing.
CO2 is a waste product from power plants that burn fossil fuels, and in the manufacture of fertilizers and cement. This waste product (and major greenhouse gas responsible for global warming) can be captured for use, for example, as fire suppressant, in carbonated beverages, food preservation, and refrigeration, but the volumes required are typically small. One potential use that involves large volumes is as an extraction solvent. Liquid CO2 happens to be a very good organic solvent for extraction; it is easily removed, and much safer for health and the environment than the usual organic solvent hexane. Liquid CO2 extraction is more selective, requires shorter extraction times and no solvent residues will be left in the product so it is easier to obtain organic certification.
Yields and properties of oils extracted with CO2 are comparable to those extracted with hexane. Liquid CO2 extraction is already commonly used on an industrial scale to achieve better quality and taste in edible oils. One of the biggest industrial scCO2 plants for the extraction of sesame oil was built in South Korea; it has an extractor volume of 2 x 3800 litres, and a pressure of up to 550 bar (1 Bar = 0.9869 atm) is currently used. Other materials such as corn, whea tgerm, sunflower seeds, safflower seeds and peanuts have also been extracted with scCO2. Using this waste resource (CO2) in oil extraction makes petrochemical/fossilfuel-based solvents redundant.
Another abundant waste product is wheat straw from agriculture, which is usually simply burnt. Clark and colleagues propose that wheat straw can be processed in an integrated, close to zero-waste biorefinery that combines extraction with liquid CO2 and low temperature microwave pyrolysis to produce a variety of products including energy; and the final waste product CO2 can be internally recycled for extraction.
In the wheat straw biorefinery, CO2 is used to extract complex compounds including fatty acids, wax ester and fatty alcohols. Low temperature microwave pyrolysis (<200 ºC) requires less energy and produces higher quality oils and chars than conventional (high temperature) pyrolysis. The oils can be fractionated to produce either transport fuels or platform chemicals such as levoglucosan and levoglucosenone. The chars can be burnt to provide heat. The quality of the chars was improved by washing to remove most of the postassium and chlorine, which produces fouling. The economic feasibility (as well as green potential) is enhanced by integrating the technologies sequentially in a close to zero waste system, beginning with extraction, followed by a combination of biochemical and thermal processing, with internal recycling of energy and waste gases. Extraction of valuable plant chemicals prior to their destruction during biochemical and thermal treatments can significantly increase the overall financial returns.
Biochemical processing offer advantages in typically low processing temperatures and high selectivity and specificity of reactants and products generated. However, it generally requires pre-treatment of the biomass, long processing times, large amounts of space, and difficult lignin treatments; and downstream processing such as distillation may be energy intensive. Alternative thermochemical routes include gasification, pyrolysis and direct combustion to produce oils, gas, char or ash. They are fast and typically continuous systems, but non-specific, and generally require high temperatures in excess of 500 ºC. So it is best to be able to take advantage of both technologies in a flexible and efficient way. A conceptual scheme for the biorefinery is presented in Figure 1 [2].
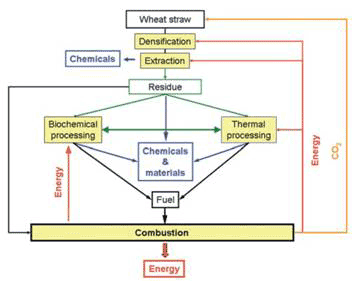
Figure 1 Wheat straw biorefinery using liquid CO2 as extraction solvent operating on a closed loop principle
Wheat straw is by no means the only waste biomass feedstock available. There is a large variety of biomass wastes containing waxes, lignins, cellulose, hemicellulose and inorganics that are ideal feedstock for a biorefinery. Agricultural residues, and in particular different kinds of straw, are a major source of available biomass in the UK. The main technologies in a biorefinery are extraction, biochemical and thermochemical processes.
Compared with traditional technologies, the team say [2], “a near to zero-waste biorefinery leads to a diverse and intricate web of products for different markets.”
In order to provide proof of concept for the scheme presented in Fig. 1, the team used wheat straw pellets (7 mm diameter) from Jackson Farms, UK, containing 10 percent moisture and a density of 1.2 g/cm3 as feedstock [2]. These were air dried and milled, and extracted for wax with scCO2 at various temperatures (40-100 ºC) and pressures (100-300 bar) for 2 h with a 40 g/min flow rate.
After that, samples of 150 to 200 g straw pellets was treated with microwave at max power of 1 200 W at 2.45 Ghz at below 180 ºC.
The first stage is the extraction of valuable compounds using scCO2 to substitute for organic solvents. The waxy cuticle of straw can be selectively extracted using scCO2, which leaves no solvent residue, so the products can be used in food, personal care or pharmaceutical applications. It has already been employed commercially for hop extraction, decaffeination and coffee and dry cleaning.
Compared with hexane, wax yields with scCO2 at 100 ºC are the same, but unwanted co-extracted contaminants such as pigments, polar lipids and free sugars are far greater in hexane. The wax extracts contain fatty acids, alkanes, aldehydes, diketones and wax esters, all having commercial applications as lubricants, food flavourings, or replacements for paraffin waxes in cosmetics. The CO2 can be recycled.
Microwave pyrolysis of the extracted wheat straw pellets yields a high quality char (30 percent) superior to those from conventional methods and with enhanced energy value, making it suitable as a coal replacement. The process also yields the following: bio-oil (20 percent) suitable for upgrading to liquid fuel, an aqueous solution of organic acids and aldehydes, an aqueous solution of sugars (35 percent), and a gas fraction (14 percent) containing combustible organic compounds (CO and methane) that could be used to produce energy.
The bio-oil is potentially one of the most valuable products of wheat straw microwave pyrolysis as it can partly replace crude oil for producing transport fuels and chemicals. The major hydrocarbon component in the microwave pyrolysis oil is 2,3-dihydrobenzofuran, and a high yield of monosaccharides such as levoglucosan. The former is useful in the pharmaceutical industry as therapeutic or prophylactic agents, as inhibitor for lipoperoxide production and cytoprotective agent. The sugars could be separated from the oil by simple water extraction into a second aqueous fraction. This opens up the possibility of taking sugars from the oil and upgrading them. Levoglucosan is attractive as feedstock for fermentation. It has been shown that levoglucosan produced by pyrolysis of cellulose can be fermented to citric acid by the fungus Aspergillus niger. Levoglucan itself has been identified as a key renewable platform molecule for more selective chemistry such as the synthesis of polysaccharides possessing biological activity, for example, anti-HIV and blood anticoagulants.
Acid base additives influence the yield of different fractions. For example, sulphuric acid treatment reduced bio-oil yield three-fold in favour of biochars, and ammonia treatment increase the char fraction by nearly 40 percent; due to polymerisation of bio-oil in the presence of acid-base catalysis. The additives also had a strong impact on the distribution of chemical compounds in the bio-oil.
Following char wash, 50 percent of potassium, 80 percent of chlorine, and 35 percent of sulphur were extracted, while only minor amounts of calcium and silicon were removed. The wash also contains decomposition products of cellulose suitable for fermentation.
The extraction using supercritical CO2 has been shown to be significantly cheaper than using hexane, chiefly as a result of savings on expensive post-extraction steps including solvent removal and purification for hexane. As part of an integrated biorefinery, CO2 captured as a product of fermentation and the processing energy from burning other fuels may also be used for extraction.
Although the capital cost of a supercritical CO2 extractor is significantly higher than hexane extraction, the lower operating costs should result in a shorter payback time, and the greener credentials of the technology should encourage investment. The microwave process is very tolerant of water compared to conventional pyrolysis and is suitable for most biomass types without pre-drying. Preliminary energy balance calculations based on the thermodynamic properties of the structural components of wheat straw during the decomposition process indicated a energy requirement of 1.8 kJ/g for microwave pyrolysis compared to 2.7 kJ/g for thermal convectional pyrolysis. Low temperature microwave pyrolysis therefore produces better quality oils and chars than conventional pyrolysis at 1.5 times the energy efficiency.
The major difficulty with Clark’s biorefinery is in finding markets for the various products. That is almost impossible without an integrated holistic approach to developing a circular economy around green, closed loop chemistry. The circular economy maximises reciprocities and cooperation as well as synergies between the different sectors [4] (Sustainable Agriculture Essential for Green Circular Economy, ISIS lecture); in the case of Clark’s biorefinery, between chemical industry and agriculture. But we must guard against the overuse of agricultural biomass for industrial processes and fuel to the detriment of food production and soil fertility (see [5] Biofuels and World Hunger, SiS 49).
Article first published 12/01/11
Comments are now closed for this article
There are 2 comments on this article.
Todd Millions Comment left 16th January 2011 09:09:02
Good post-
as well as this,some work was done in Canada in the 1990's on using liquid co2,as a drycleaning fluid,with obvious benifits on the tox residue/leaks axis.Too boot operating costs and effectiveness were shown too be superior in tests.Such a pity our bank and refinery mafias had already invested so much in the toxic solvent methods(including the health and enviroment minister and mandarin bribes).
I have tried chocolate that was defatted with liquid co2,instead of hexane-it was very good,and I'm told that the grade of the product is higher than hexane extracted from the same coca stocks.
Good point as well on other uses for straw and other crop'waste'stocks(see; ecoblock for instance.).
Rory Short Comment left 14th February 2011 23:11:37
The contents of this article are music to my ears. This is the way we should have been trying to do things all along. The trouble was, and is, that the linear approach as opposed to the circular approach usually yields quicker monetary profits especially if the ecological consequences of this approach are ignored and left for somebody else to bear.