Harvesting solar energy with a specially designed metal complex that splits water into hydrogen and oxygen with novel chemistry and regenerates itself. Dr. Mae-Wan Ho
Splitting water is one of the ways to harvest sustainable energy from the sun, especially in creating hydrogen which can burn as clean fuel [1] ( Harvesting Energy from Sunlight with Artificial Photosynthesis , SiS 43). This imitates the natural process of photosynthesis in green plants that use sunlight to abstract hydrogen from water (to fix carbon dioxide into carbohydrates and ultimately biomass), liberating oxygen. Despite a great deal of effort, nothing practical has emerged so far.
David Milstein and his research group at Weiszmann Institute's Organic Chemistry Department in Israel have now found a new way of splitting water altogether [2]. This involves the liberation of hydrogen and oxygen in consecutive heat and light driven steps, catalyzed by a single organic metal complex of the element ruthenium that the team has designed, in which the metal centre and the organic part attached to it cooperate in splitting the water molecules much more easily, it seems.
The team found that on mixing the ruthenium complex with water, the bonds between the hydrogen and oxygen in water break, with one hydrogen atom ending up binding to the organic part, while the hydroxyl group (OH) binds to the metal centre. When the water is heated to 100 C, hydrogen gas is released from the complex, and another OH group attaches to the metal centre.
The most interesting part is the third light stage, says Milstein. “When we exposed this third complex to light at room temperature, not only was oxygen gas produced, but the metal complex also reverted back to its original state, which could be recycled for use in further reactions.”
Most remarkable is the generation of a bond between two oxygen atoms (of the two OH groups) promoted by the metal complex, and it has been unclear how it could take place. But Milstein and his team have worked out the new mechanism. Using a combination of heavy isotopes especially of H and O in water, they were able to track the actually molecular reactions involved [3]. These experiments showed that during the third stage, light provides the energy required to cause the two OH groups to get together to form hydrogen peroxide (H2O2 ), which then quickly breaks up into oxygen and water. The team provided evidence that the bond between the two oxygen atoms required in forming H2O2 is generated within a single molecule; that is, from a the two OH groups attached to a single metal centre, and not between oxygen atoms residing on separate molecules from different metal centres.
A simplified version of the usual photosynthesis-based water splitting scheme (Figure 1, upper half) involves the absorption of a photon (h n ) by the light-sensitive pigment, or chromophore (C), exciting an electron (e-) from its ground state to initiate the process of charge separation and charge transfer. The excited electron travels to an electron acceptor (A), leaving behind a positive hole that is filled by the electron donor (D). Proton reduction and hydrogen evolution occurs at the catalyst Catred , which accepts electrons, and water oxidation and oxygen evolution occurs at the other Cat ox , which accepts holes [4]. Because the electrons and holes separate, water splitting is really two half reactions that either consume or generate electrons. Each half-reaction can be investigated and optimized independently, and research successes have been reported for both. But inevitably, a ‘sacrificial' electron donor or acceptor is consumed in the reaction that is not optimised. Although H 2 has been generated by light induced chemical reactions, the efficiency and durability of the systems are still far from practical. The formation of O2 is even more difficult (but see [5] Making Fuel from Water , SiS 43, for the latest in getting oxygen from water)..
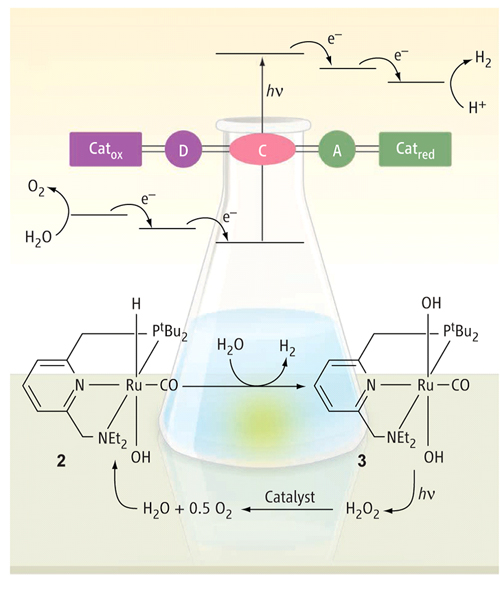
Figure 1. Comparison of photosynthesis-based water splitting with that of Milstein and coworkers (redrawn from [3])
The organic ruthenium complex designed by Milstein's group works differently altogether. It depends on the reversible addition and subtraction of a proton (H + ) to an arm of the ‘pincer' ligand holding the ruthenium ion. This usually difficult process is made possible through aromatization and dearomatization of the central ring of the pincer ligand. (This refers to the six-member ring in the aromatic form with three alternating double bonds inter-converting with one that has only two double bonds.) In the presence of water, protonation of the ligand arm, and binding of OH to the Ru metal centre takes place (see Fig. 2).
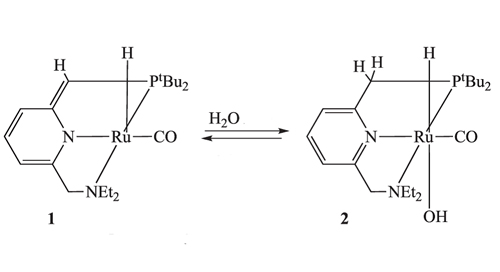
Figure 2. Ruthenium complex 1 is protonated by H2O to complex 2 , in which the OH group is coordinated to the ruthenium centre
The added proton becomes a captive source of H+ that can react with the ruthenium bound hydride (H- ) to give H2 gas, which is also an unusual reaction.
H + + H - ? H2 (1)
Ru complex 2 , with hydride and hydroxide ions bound (RuH(OH) can react with another H2O molecule (see lower half of Fig.1). This reaction proceeds on heating to liberate H2 .(as in reaction (1) above) and subsequent addition of water to form the dihydroxide complex 3 (Ru(OH)2 .
On exposure to light, Ru(OH)2eliminates the hydrogen peroxide. Rapid reaction of 2H2O2into O2and 2H2O follows, completing the process of splitting water. This reaction also regenerates the ruthenium complex 1 which reacts with water to give complex 2 , and so on to start another cycle.
The water-splitting scheme of Milstein and his team still has some way to go before it can be practical. “The project is directed, in the long run, for efficiently producing hydrogen from water using visible light, and without using sacrificial chemicals.” David Milstein told me. But the fact that a relatively simple molecular system can split water with mechanisms not previously conceived has excited the water-splitting community.
Article first published 06/07/09
Comments are now closed for this article
There are 4 comments on this article.
Li Kangmin Comment left 2nd October 2009 16:04:42
I wonder if you would like to introduce this new fatastic innovation to Chinese people. If so please send me figures.
Li Kangmin
Torben Munk Nielsen Comment left 7th July 2009 08:08:25
Thank you for all your are doing, Dr. Mae-Wan Ho
Love and light Torben
Rory Short Comment left 7th September 2009 04:04:45
How absolutely fascinating. It is breakthroughs like this that open the way for us to save ourselves. That is if we actually want to do so.
tom Comment left 7th February 2013 20:08:28
how complex is this procedure and how large of a space would you need for it imagine if it could be attached to a vehicle that used hydrogen as fuel by fusing with oxygen to create water a car that could be self sustainable would save fossil fuels the possible use in powerplants and severe drop in emiisions