Water and Fire
Burning water is a way of life for all organisms, in animals as in plants, with a little help from sunlight perhaps Dr. Mae-Wan Ho
Can organisms like animals and humans burn water? Burning or oxidising water is what green plants do with the greatest of ease. They depend on absorbing energetic photons from sunlight to make water give up electrons. A great deal of current effort ia dedicated to artificial photosynthesis [1] (Harvesting Energy from Sunlight with Artificial Photosynthesis, SiS 43), and oxidising water (H2O) to oxygen (O2) is certainly half of the reaction; the other half is the production of hydrogen (H2) that can actually be burnt as fuel.
But can other organisms such as animals and human beings do the same? Not in that way, but they burn water all the same. They oxidize water, not to get energy as much as to create exquisitely targeted local weapons for killing bacteria and other microbes. (The recent discovery that the water in cells and tissues of living organisms may be charged up by exposure to infrared light suggest that animals too, may depend on sunlight to energize themselves [2] (Water Electric, SiS 43). I have often wondered why I feel so energetic after sunbathing.)
Some years ago, a team of Californian researchers discovered the surprising ability of antibodies produced by the immune system to create highly reactive chemicals that cells can use to cleanse themselves and poison invaders, by burning or oxidizing water. Chemists Richard Lerner and Paul Wentworth at the Scripps Research institute of La Jolla, stumbled upon this new role of antibodies by accident [3]. They found antibodies generating H2O2 (hydrogen peroxide) when exposed to UV light. They tested numerous other antibodies and the result was the same each time. The antibodies were generating H2O2 from singlet oxygen (1O2), an energetic and highly reactive form of the oxygen molecule produced when a source of energy, such as UV light excites the ordinary triplet oxygen (3O2), catalyzed by the antibody.
Singlet oxygen and hydrogen peroxide belong to a class of molecules called reactive oxygen species (ROS) that have become the subject of much research effort in recent years (see Box).
Reactive oxygen species (ROS)
Reactive oxygen species are small molecules that contain oxygen and are more reactive than ordinary molecular oxygen because they have been activated from the ground state, some are free radicals, containing an unpaired electron. ROS include singlet oxygen, superoxide, peroxide, hydroxyl radical and hypochlorite ion.
The outer electron structures of ROS are represented below on the right. For comparison, the ordinary ‘non-reactive’ oxygen species are given on the left.
| Non-ROS | ROS |
| Triplet oxygen ∙O-O∙ | Singlet oxygen O-O: Superoxide ∙O-O:- Perhydroxyl radical ∙O-O:H Hydrogen peroxide H:O-O:H Hydroxyl radical H:O∙ |
| Hydroxyl ion H:O:- | Hypochlorite ∙O-Cl:- |
| Water H:O:H |
Ordinary triplet oxygen is a bi-radical as its two outer electrons are unpaired and in parallel spin; it is in the lowest energy ground state. It can become activated by receiving enough energy to reverse the spin of one of its unpaired electrons. When that happens, it will form the activated singlet oxygen in which the two electrons have anti-parallel spins and become paired. Singlet oxygen can participate in reactions involving the simultaneous transfer of two electrons, making it more reactive.
The second way to activate triplet oxygen is to acquire a single electron to form superoxide ion (which has a negative charge), and in turn the perhydroxyl radical (uncharged, but with an unpaired electron), hydrogen peroxide, and the hydroxyl radical (again uncharged, and with an unpaired electron). Another common ROS is the hypochlorite ion, which is also a radical.
ROS are formed in the body as the result of several different mechanisms.
1. Interaction of ionizing radiation with biological molecules
2. From electron transport during cellular metabolism
3. Synthesis by enzymes in phagocytic cells such as neutrophils and macrophages which use the ROS to kill bacteria and other microbes
4. Synthesis by similar enzymes in all tissues and cells for signalling purposes, as recently discovered .
ROS, especially those that are radicals with unpaired electrons can be very damaging to cells as they react with other molecules to gain a stable configuration of paired electrons, they convert their target molecule into a radical, so a chain reaction begins that will propagate until two radicals meet to form a covalent bond. In the process, molecules can become cross-linked, deforming the structures, such as membranes of which the molecules are part.
Cells have a variety of defences against ROS. These include superoxide dismutase enzymes, which convert two superoxide anions into a molecule of hydrogen peroxide and one of oxygen, and catalases, which decompose hydrogen peroxide into oxygen and water.
The difference in energy between triplet and singlet oxygen is about 1 eV, well below the energy of the UV photon absorbed by the protein, which can then be transferred to the triplet oxygen. But where do the electrons come from to reduce singlet oxygen to H2O2? At first, the researchers thought the reaction was burning the protein itself to produce H2O2, but far too much H2O2 was produced for that to be the case. The production of H2O2 by antibodies was sustained for long periods under UV light, remaining linear up to more than 40 mol equivalent of H2O2. When the H2O2 was removed, the antibodies resumed H2O2 production at the same initial rates, and remained practically unchanged even after 10 cycles of UV irradiation followed by removal of H2O2 [4].
After eliminating other sources for the hydrogen peroxide, such as Cl- and metal ions, the team hit on the idea that the singlet oxygen was reacting with, or burning water to give H2O2. In the presence of singlet oxygen, UV irradiation of antibodies leads to incorporation of oxygen from water into H2O2. By tagging water with a heavy isotope of oxygen, Lerner’s team confirmed that oxygen in H2O2 did indeed come from water, in the ratio of about 1:2.2.relative to molecular oxygen according to the scheme:
2 1O2 + 2 H2O -> 2H2O2 + 3O2 (1)
The Scripps researchers teamed up with reaction modelling expert William Goddard III of the California Institute of Technology in Pasadena and his student Xin Xu. They suggested that a water molecule could combine with the singlet oxygen to produce hydrogen trioxide (H2O3), which eventually rearranges to produce H2O2.
Because of energy barriers, the initial reaction of water and singlet oxygen probably would never take place on its own. But Xu and Goddard calculated that if the reaction started with at least two water molecules, one of them would act as a catalyst driving the reaction forward. It decreases the activation energy barrier from 64.7 kcal/mol to 31.2 kcal/mol if two water molecules were involved; and to 12.0 kcal/mol if 3 water molecules were involved. The reverse reactions have energy barriers of 19.2 kcal/mol and 0 kcal/mol respectively, which suggest that H2O3 is not stable in bulk water. The proposed reactions would go as follows: the singlet oxygen reacts with water to form H2O3 and a dimer of H2O3 then rapidly rearranges to give H2O2 and oxygen.
H2O + 1O2 -> H2O3 (2)
2 H2O3 -> 2 H2O2 + 3O2 (3)
But getting that precise arrangement of water molecules and singlet oxygen to come together is not easy. Perhaps the antibody has a role? To find out, Wentworth and Lerner turned to Ian Wilson, an X-ray crystallographer at Scripps. Using high resolution X-ray Wilson made electron-density maps of the atomic structure of four different antibodies [5]. Scrutinising the regions that all antibodies share in common, Wilson’s group identified three sites close together that could bind oxygen molecules as well as neighbouring sites capable of holding water molecules in the right places.
Isotopic labelling confirmed that the antibodies play a catalytic role in converting 1O2 to H2O2.
These results were confirmed by another research team at California Institute of Technology, Pasadena [6] using quantum mechanical calculations. The proposed reaction intermediates, HOOOH (H2O3) and its dimer are stabilized at the ‘Greek key’ interface of the light and heavy chains, unique to antibodies and T cell receptors, and not present in b2-microglubulin. This was consistent with the experimentally observed lack of HOOH (H2O2) production in the latter as opposed to the former. The catalytic site at which these reactions take place have ordered water clusters (dimers and trimers) which enables 1O2 to react with multiple water molecules. In contrast, the binding site for the product, H2O2 does not contain any ordered water clusters, and is in a buried hydrophobic pocket.
Further studies comparing the UV irradiated and native structure of the antigen-binding fragments revealed specific modifications to amino acid residues tryptophan L163 and glutamine H6 located in the interfacial regions of the previously identified antibody catalytic site [7].
Subsequently, the Scripps Institute group found evidence that in addition to H2O2, ozone (O3) [8] or other trioxygen species [9] are also produced and released by antibodies, which account for the powerful bactericidal properties of all antibodies, regardless of antigen specificity.
These discoveries are all the more surprising because antibodies have long been cast in the role of “semi-combatant” in the immune system’s war against invading microbes [10]; for it is the immune cells such as macrophages that actually kill bacteria by engulfing them in phagocytosis and digesting them in the vacuoles or phagosomes formed within the cell.
Neutrophils, the most abundant white blood cells in the bloodstream, are also the main phagocytes of the immune system. They kill bacteria and fungi, partly by triggering an oxidative burst composed of a set of enzymatic and chemical reactions that result in the formation of ROS. The first step in this cascade, the reduction of two oxygen molecules, is initiated by the enzyme NAD(P)H oxidase, which becomes assembled in the membrane when the cell is activated by antibody-coated bacteria. The oxidase produces peroxide anion (O2* - )
NAD(P)H + 2 3O2 -> NAD(P)+ + H+ + 2O2*- (4)
The reactivity of O2*- with the cell is relatively low. Its function is to generate more powerful ROS. Its key reaction within the phagosome are either to acquire a proton to become a hydroperoxyl radical (HO2*), followed by a fast rearrangement (dismutation) into H2O2 and 3O2, with or without the help of superoxide disumutase enzyme. The H2O2 is then used to oxidize Cl- to hypochlorite OCl-, a reaction catalyzed by myeloperoxidase. Hypochlorite is highly bacteriocidal but is also used to form chloramines, some of which are even more microbicidal than hypochlorite. An alternative fate of H2O2 is conversion into a hydroxyl radical (OH*).
Given that H2O2 and HOCl are present at high concentrations within the phagosome, there is the possibility for a chemical production of high levels of singlet oxygen 1O2.
H2O2 + HOCL -> 1O2 + HCl + H2O (5)
As antibodies can catalyze the formation of H2O2 from 1O2 and H2O via the intermediate of H2O3, with the production of ozone, the Scripps researchers looked for evidence that neutrophils might also produce ozone, and they were successful [11]. The scheme they proposed for the generation of ROS in neutrophils is given in Fig. 1.
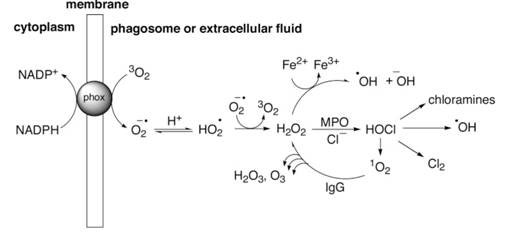
Figure 1. Reactive oxygen species generated by neutrophils
That is not the end of the story. In 2008, a team of Japanese researchers led by Kouhei Yamashita at Kyoto University found that four amino acids (without antibodies) – tryptophan, methionine, cysteine and histidine – are also able to catalyze the conversion of singlet oxygen to ozone when exposed in aqueous solution to UVA radiation. Other water soluble amino acids tested did not have this ability [12].
Indeed burning water seems rather easy, it is a way of life. Animals including human beings do it all the time for signalling purposes and to regulate the energetic status of tissues and cells [13] (Living with Oxygen, SiS 43).
Article first published 17/06/09
Comments are now closed for this article
There are 4 comments on this article.
Warren Brodey M.D. Comment left 18th June 2009 05:05:35
I would like to forward a copy of this to Vilhelm Schjeldrup M.D who has done clinical studies of singlet oxygen giving this to children with chronic asthma . HIS STUDY PRODUCED GOOD RESULTS. But were not given much attention by those who can finance further research. He has collaborated with other studies providing singlet oxygen to plants. His emailaddress is vilsch@online.no
Mae-Wan Ho Comment left 28th July 2009 03:03:31
Hi Warren Brody,
Please forward to Vilhelm. Would love to hear from him regarding his trial and why he thought the children with asthma could benefit from singlet oxygen.
Radoslav Bozov Comment left 9th January 2010 03:03:59
Burning water is entropic force, thus reducing oxygen to hydrogen peroxide is negentropic force, further reducing it to water is negentropic force, while oxidizing carbohydrates by using peroxide is entropic force. Evolution is the result of these two opposing forces.
mzm Comment left 4th February 2010 23:11:13
O3 is an asthma trigger