Waste-gobbling bacteria may be our dream ticket to clean renewable energy Dr. Mae-Wan Ho
Bacteria that gobble wastes are a godsend. They prevent the build up of wastes in our environment and play an indispensable role in making wastewater safe for domestic animals, wild life, and human beings. In many Third World countries, these same bacteria are working miracles turning manure and other wastes into valuable resources to support highly productive farms that require no input and generate little or no waste ("Dream farm", this series). When these bacteria are confined in anaerobic digesters with limited or no access to oxygen, they ferment the wastes, release and conserve nutrients for livestock and crops, and produce 'biogas' as by-product, which typically consists of about 60% methane (CH4) and a small amount of hydrogen (H2), both of which can be burnt as smokeless fuel.
Within the past two years, these same bacteria are showing even more remarkable potential for producing clean and renewable energy while reducing greenhouse gas emissions.
The "hydrogen economy" is on everyone's lips as the answer to the ultimate clean energy. Burning hydrogen produces pure water instead of green house gases, and it is by far the most energetic fuel on earth, weight for weight. But in order to really reduce green house gas emissions, hydrogen must be produced sustainably with renewable sources such as sun, wind and biomass. About half of all hydrogen produced currently is from natural gas, the rest is produced primarily using other fossil fuels. Only 4% is generated by splitting water using electricity derived from a variety of sources.
At BIOCAP Canada's First National Conference in February 2005, a research team at the Wastewater Technology Centre and the University of Waterloo in Ontario, Canada, presented a poster describing a prototype process for producing substantial amounts of hydrogen as well as methane from potato waste [1].
The team used a two-stage anaerobic digestion to get first hydrogen and then methane. In this way, it was possible to optimize the first stage for producing hydrogen. The key appears to be an acidic pH of 5.5 in the hydrogen reactor, instead of pH 7 in the methane reactor. Both reactors were run at 35C.
They pulped the potatoes bought from a store and treated the slurry with peptone (an enzyme that breaks down protein), then seeded the two reactors - one for hydrogen the other for methane - with digested sludge from the local wastewater treatment plant to get the bacteria in place. For the hydrogen reactor, the seed sludge was pre-cultivated in a sucrose medium for a few days before switching to potato waste when high hydrogen production was confirmed. For the methane reaction, no precultivation of the sludge was required.
From the 4th day, the potato pulp replaced sucrose and hydrogen biogas was produced continuously for a further 90 days. The maximum production rate from the one litre reactor was 270ml/h on the 17th day, and the average rate over the entire 90-day period was 112.2ml/h. The hydrogen fraction fluctuated between 39 and 51 percent of the biogas (v/v). The average chemical oxygen demand (COD) concentration (a measure of the amount of waste present) of the fluid coming out of the hydrogen reactor was 7 220mg/L, at an input concentration of 12 800mg/L. So more than 40 percent of the waste was removed.
Once hydrogen production became stable after the 20th day, the outflow from the hydrogen reactor was transferred to the second, bigger (methane) reactor, 5 litres in volume. During the 70 days of operation, methane biogas was produced continuously; the maximum rate was 410ml/h, and the average rate, 213 ml/h. The concentration of methane in the biogas was between 69 and 79 percent. The average COD concentration in the methane bioreactor outflow was 4 130 mg/L. Again, the process removed more than 40% of the wastes. Together, the two reactors removed 68% of the waste.
Based on the hydrogen and methane production rates, the average energy yield from each kilogram dry weight of potato waste was 4.96 MJ (1.4kWh) and the maximum energy yield, 9.58 MJ (2.7kWh). For comparison, burning 1 kg wood yields about 20MJ [2]. But because the energy is generated from waste, it is essentially free, and does not require chopping down trees.
Potato is the third largest food crop in the world, and Canada is one of the leading producers (4.7million tonnes annually). Large amounts of potato waste come from food and potato processing plants. This is potentially a huge source of renewable, clean energy.
A research team in Pennsylvania State University has also discovered how to coax the same bugs to make plenty of hydrogen while they are gobbling wastes [3].
When the bacteria ferment glucose, they generate a maximum of 4 molecules of hydrogen per molecule of glucose and end up at best with two molecules of acetic acid that they cannot convert further to hydrogen due to an electrochemical barrier. But, given a little electrical boost, the bacteria can jump over the barrier to generate more hydrogen.
The research team, led by Dr. Bruce Logan, already made news in 2004 [4], when they succeeded in getting the bacteria to produce electricity while removing wastes.
The bacteria were put into a microbial fuel cell that generated 26mW m2 of electricity while removing up to 80% of the wastes that flowed through.
These waste treatment bacteria, numerous species belonging to many genera including Geobacter, Shewanella, and Pseudomonas, have the ability to transfer electrons obtained by fermenting wastes to external metals [5]. When the bacteria are attached to electrodes, the electrons are transferred to the electrodes (the anode), to flow through an external circuit to the cathode where they combine with oxygen from the air and protons (hydrogen ions) to form water.
The reactor then used was a single cylindrical plexiglass chamber the size of a soda water bottle in which the anode, consisting of eight graphite rods, was placed in a concentric arrangement surrounding a central cathode that was exposed to air. The air-porous cathode consisted of a carbon/platinum catalyst/proton exchange membrane layer fused to a plastic support tube.
The efficiency of the system, based on waste removal and current generation was less than 12%, indicating that a substantial fraction of the organic matter was lost without generating current; perhaps in producing more bacteria. But as the bacteria were doing their intended job, which was to remove waste, any electricity generated at the same time was an extra bonus.
Now, the team has discovered that by excluding air from the cathode, and by giving the bugs a boost of about 250mV, they can make the bugs produce hydrogen at high efficiency. They refer to this process as electrochemically assisted microbial production of hydrogen.
Normal fermentation converts glucose to dead end products such as acetic and butyric acid:

In the first case, four molecules of hydrogen are generated, and in the second, only two molecules. The greatest theoretical yield possible is four molecules of hydrogen per molecule of glucose.
The microbial fuel cell, however, offers a new solution to the problem. By augmenting the electric potential in the microbial fuel cell circuit, it gave just the little help needed for the bacteria to make hydrogen out of acetic acid.
In a typical fuel cell, the open circuit potential of the anode is about -300mV. If hydrogen is produced at the cathode, the half reactions occurring at the anode and the cathode with acetic acid oxidized at the anode, are as follows:
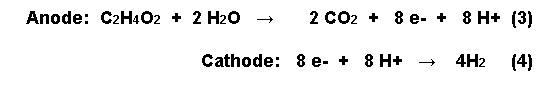
In order for the bugs to donate electrons to the anode from acetic acid, however, the anode potential has to be made less electronegative.
To improve the efficiency of the intended process, the researchers also created a two chamber microbial fuel cell instead of the one-chamber version they had previously constructed. One chamber contained the anode, the other the cathode, separated by a proton exchange membrane. A major advantage of housing anode and cathode in separate chambers is that the hydrogen produced at the cathode is separated from the carbon dioxide at the anode at source. Instead of being exposed to air, the cathode chamber was sealed. A voltage of 250mV or greater was applied to the circuit by connecting the positive pole of a power supply to the anode, and the negative pole to the cathode.
The external power supply increased the anode potential from -300mV to -291mV with a boost of 250mV and to -275mV with a boost of 850mV, producing hydrogen and degrading more than 95% of the acetate in the process. The recovery of electrons as hydrogen was over 90%. The Coulombic efficiency - defined as the recovery of total electrons in acetate as current - ranged from 60 to 78% depending on the applied voltage. Thus 2.9 of the theoretical maximum 4 molecules of hydrogen are obtained from the acetic acid reaction with water by an injection of 250mV of electricity (see equation 3). This compares favourably with the costly1800-2000 mV needed for getting hydrogen from splitting water [6].
A combined fermentation and bioelectrochemically assisted anaerobic microbial fuel cell has the potential to produce as much as 8 to 9 molecules of hydrogen starting from a molecule of glucose (The theoretical maximum is 12, see equations 1, 3 and 4.)
With this bioelectrochemically-assisted reactor, hydrogen can be produced from any type of biodegradable organic matter. Combined hydrogen production and wastewater treatment will offset the substantial costs of wastewater treatment as well as provide a contribution to the hydrogen economy. As the technology is rather simple, it can be adapted for use at different scales, in third world countries as well as industrialised countries.
At the BIOCAP Canada conference referred to earlier, another poster pointed out that 45 of 56 wastewater treatment plants in large urban areas of Ontario, Canada incorporate an anaerobic digestion process to reduce the volume of disposable sludge; but the methane produced is mostly wasted by being flared off to the atmosphere. A conservative estimate suggests that if all the wastewater sites were to use anaerobic digesters and simply recover the methane to generate electricity, this would produce 1.51 GWh/day [7]. It was a small percentage of the total of 317GWh consumed each day in Ontario. But on average, 0.3kg of CO2 is emitted per kWh energy produced from Ontario Power Generation, so simply recovering the biogas energy from the current sites using anaerobic digesters represents a saving of 432 tonnes of CO2 per day.
Imagine what can be achieved if waste treatment were optimised for hydrogen production.
Article first published 03/06/05
Comments are now closed for this article