The GM oil seed rape varieties in UK's farm scale evaluations are terminator crops with in-built sterility to protect patented crop genes. Terminator crops like these had been vigorously rejected by farmers all over the world as it goes against their right to save and replant seeds. These crops are now being considered for commercial release in Europe. Dr. Mae-Wan Ho and Prof. Joe Cummins tell us why these crops carry unique risks for health and biodiversity, and should on no account be approved.
'Terminator technology' is so named by its critics because it genetic engineers sterility into crop plants, for no other purpose than to protect and enforce corporate patents on GM seeds.
The public first became aware of the technology in patents jointly owned by US Department of Agriculture and Delta and Pine Land Company. There were massive protests worldwide, and Monsanto, which acquired the Delta and Pine patent rights, backed down from developing the terminator crops described in that particular patent. However, as we were to learn, there are many ways to engineer sterility. It is not easy to find the information, as very little is published, and applications for field trials or commercial approval routinely conceal such information from the public under 'commercial confidentiality'. All our requests to regulatory bodies for details on specific constructs were ignored.
MWH first became aware that the OSR varieties in Britain's farm scale evaluations (FSE) are terminator crops in December 2000. As an expert witness defending citizens who had taken action against the FSE in Scotland, she gained access to AgrEvo's (later Aventis, now Bayer CropScience) application for field trial [1, 2]. The document mentioned the unmistakable male sterile system that belongs to terminator technology, but it gave away few details on the actual transgenic constructs used.
Coincidentally, we were preparing a submission [3] to a public consultation document, "Guidance on Best Practice in the Design of GM Crops" put out by the UK Government's Advisory Committee for Release to the Environment (ACRE). One of the main 'enabling technologies' for 'best practice' - to prevent gene flow - as suggested in the document, is to engineer seed or pollen sterility. The technology was promoted simultaneously in the United States. The USDA solicited public comment on the technology, also with the recommendation that it could be used to prevent gene flow.
By then, we discovered that terminator crops have been field tested in Europe, Canada and the US since the early 1990s, and several were already commercially released in North America.
We ploughed through numerous patents to find the many ways in which sterility could be engineered into crops and alerted our regulators [2, 4], to no avail.
The precise construct differs from one crop to another, add to that the inherent uncontrollability of the technology, which generates 'event-specific' characteristics that are simply not documented, and/or kept hidden from the public under 'commercial confidentiality'. Our current reconstruction is made by piecing together clues from a number of documents including especially the recently released report on gene flow [5] (see Figure below). It supersedes our earlier attempts [2,3].
The male sterility system in these GM OSRs consists of three lines.
The male sterile line is maintained in a 'hemizygous' state, ie, with only one copy of the male sterility gene, barnase, linked to glufosinate-tolerance gene, H. The barnase gene is driven from a promoter (gene switch) that's active only in the anther or male part of the flower. The expression of the barnase gene in the anther gives rise to the protein barnase, an RNAse (enzyme that breaks down RNA), which is a potent cell poison. The cell dies and stops anther development, so no pollen is produced. This male sterile line is probably perpetrated in the hemizygous state by crossing to a non-GM variety, and using glufosinate-ammonium to kill off half the plants in the offspring generation that do not have a copy of the H-barnase transgene.
The male restorer line is homozygous (with two copies) for the sterility-restorer gene, barstar, also linked to glufosinate-tolerance gene H. The barstar gene is also placed under the control of the special promoter that's active in the anther. Its expression gives the barstar protein that's a specific inhibitor of barnase, thereby neutralising the latter's activity.
The important information here is that the barstar and barnase genes are on different chromosomes and each is linked to a glufosinate-tolerance gene, probably the same one, H.
Crossing the male-sterile line to the male-restorer line produces a F1 hybrid, which contains two kinds of plants. One has both the H-barnase and H-barstar transgenes in hemizygous state, in which the barnase is neutralised by barstar, thus restoring anther development to produce pollen. The other has only H-barstar in hemizygous state. Both kinds of plants are male-fertile as well as glufosinate-tolerant.
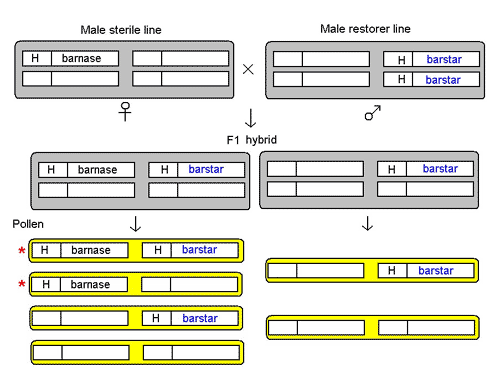
Figure 1. Reconstruction of the male sterile system devised by PGS, now Bayer CropScience, See text.
The two kinds of F1 hybrid plants produce different kinds of pollen. As H-barnase and H-barstar are on different chromosomes, they assort independently of each other. The plants with H-barnase and H-barstar both in a hemizygous state produce four kinds of pollen in equal proportions, three of which carry the glufosinate-tolerance gene. The plants with only H-barstar in hemizyous state produce two kinds of pollen in equal proportions, those with the glufosinate-tolerance gene and those without. Thus, 5/8 of the pollen produced by the F1 hybrid plants will spread the glufosinate-tolerance gene; and 2/8 of the pollen will spread the male-sterility gene barnase, half of them carrying the male-sterility restorer gene barstar, and half without.
Gene flow - drastically underestimated
The most obvious hazard is gene flow to non-GM varieties of OSR and to wild relatives. The National Institute of Agricultural Botany (NIAB) was commissioned by the government to estimate the spread of transgenes from GM crops. The report [5], released by the Food Standards Agency (FSA) just before Christmas, created a stir. Levels in excess of 0.5% cross-pollination were found in some samples of the neighbouring conventional OSR taken at 200m from the GM OSR source at one FSE site. The report states (p.3), "There may be a need to review isolation requirements in keeping with current legislation on contamination thresholds in crops, in light of this research."
A careful reading of the report reveals that things are considerably worse than the data suggest. If anything, this experiment appears to be designed to underestimate gene flow, so the true extent could be considerably higher, as we shall show later. This is reminiscent of another FSA-commissioned research that found horizontal gene transfer in the human gut even though it was designed to stack the odds against detecting such events [6].
In view of ACRE's recommendation that terminator crops could be used to prevent gene flow, the detection of such substantial gene flow must be quite a blow.
The researchers monitored the spread of glufosinate-tolerance from the F1 hybrid to conventional OSR. As explained, only 5/8 of the pollen released carry the glufosinate-tolerance gene, so gene flow is underestimated by 37.5% from this factor alone, as the report points out. But that's not the only source of underestimate.
Apparently, a small proportion of plants in the F1 may also be male-sterile (usually 8-9%) (p.3), and OSR is predominantly self-pollinating. Outcrossing rate can vary between 12% and 47% depending on geographic location, weather conditions at time of flowering, and within-plant position of the flowers. Among flowers at different positions on the same plant, outcrossing varies from 11% at the top of the inflorescence to 39% at the bottom (p.23).
Thus, the rates of cross-pollination detected in the experiment are 7% to 36% of those that would be found in the case of fully fertile, out-crossing crops. (These values are obtained by multiplying together the fraction of pollen with herbicide-tolerance gene, the fraction of plants that produce pollen (Non male-sterile), and the outcrossing rate, ie 0.625 x 0.92 x 0.47 = 0.36 for the upper limit, and 0.625 x 0.91 x 0.12 = 0.07 for the lower limit.)
The spread of herbicide tolerance gene, apart from contaminating neighbouring crops, has the potential to create herbicide tolerance weed. But more insidious effects may come from the male-sterility gene to which the herbicide tolerance gene is linked.
As one-quarter of the pollen from the GM F1 hybrid contains the barnase gene, the male sterile trait could be directly transferred by pollen to non GM OSR as well as wild relatives. This could severely compromise the agronomic performance of conventional crops and cause wild relatives to go extinct. The effects are not just limited to the male-sterility trait itself, but transgenic instability associated with the constructs (see below).
There are also immediate impacts on health.
Barnase is a ribonuclease (RNAse), an enzyme that kills cells by breaking down RNA indiscriminately. The gene is isolated from the soil bacterium, Bacillus amyloliquefaciens, which also produces the specific inhibitor of barnase, barstar. Barstar binds to barnase and inactivates the enzyme.
Barnase, unaccompanied by its specific inhibitor barstar, is known to be a potent cell poison [7]. Traces of barnase are toxic to the rat kidney [8] and to human cell lines [9]. Barnase is actually being exploited as a conditional 'suicide gene' to cause cell death in mammalian [10] and human [11] cells when it is induced.
As fully one-quarter of the pollen produced by the GM F1 hybrid OSR actually contains the barnase gene, and half of that without the barstar gene, it raises serious questions concerning the activity of the barnase gene and its risks to health and biodiversity.
Is barnase expressed at low basal levels in the plant tissues when it is not in the induced state? Are the constructs sufficiently stable to ensure that the barnase is only active in the anther? Barnase, even if expressed at low levels could prove toxic to a wide range of animals that interact with the plant, including not only human beings, but also small rodents and bees. It could also enter the human food chain in bee honey.
The ecological and health impacts of the horizontal transfer of transgenes to bacteria, animal and human cells have not been assessed. Barnase transferred to a pathogenic bacterium could potentially increase its armoury of bio-weaponry against its victims. If transferred to human cells, it has the potential to cause cell-death.
As stated in an earlier submission to the GM Science Review [12] transgenic instability is well known. There are no data to document the stability of these GM OSR varieties (or any other GM variety currently under field trial or commercial release).
There are, in addition, specific features of the construct expected to increase transgenic instability. One of these is the duplication of H (glufosinate tolerance) on separate chromosomes.
Duplicate H copies on separate chromosomes could lead to translocations (moving part of one chromosome to another) originating from inappropriate pairing and recombination at the homologous H genes.
The peculiar genetics of OSR is a further complication. It is an allotetraploid consisting of two different sets of chromosomes A and C duplicated (AACC, 2n = 38). The presence of H on non-homologous chromosomes could lead to largescale genome scrambling and genetic instability. Duplication of H could also result in gene silencing.
There is evidence obtained by the local group in Munlochy of a massive breakdown in glufosinate tolerance in their local GM OSR crop [13], which has never been explained by the company.
The spread of these constructs to conventional crops and wild relatives could instigate similar genetic instabilities, leading to catastrophic breakdown.
We simply have no data to assure us that this has not happened or cannot happen.
Other terminator crops make use of site-specific recombinases that are known to scramble genomes [14, 15], and are perhaps the most dangerous tool in the terminator repertoire.
Article first published 20/02/03
Comments are now closed for this article