Graphene can be synthesized in large qualities and with minimum environmental impacts from methane and also carbon dioxide, thereby also saving the climate Dr. Mae-Wan Ho
Industrial production of graphene has already begun in China (see [1] Graphene and the New Age of Carbon, SiS 59), though the precise methods used in production are not known. My choice is for making it from greenhouse gases in an environmentally sustainable way.
Current methods that can mass produce graphene are chemical exfoliation of graphite, epitaxial growth on silicon carbide (SiC) and chemical vapour deposition on transition metal foil. Chemical exfoliation requires harsh chemicals such as potassium permanganate (KMnO4) in concentrated sulphuric acid (H2SO4), and hydrogen peroxide (H2O2) to oxidize graphite and separate the layers to make a colloidal dispersion of graphene oxide, which is then reduced back to graphene with highly toxic chemicals such as hydrazine [2]. Epitaxial growth on SiC requires heating SiC to high temperatures in excess of 1 100 ºC in an atmosphere of argon and the quality and size of the product depend on the SiC wafer, which makes the process quite expensive as well as energy intensive [3]. Chemical vapour deposition (CVD) on transition metal foil looks the most promising, producing
single layer graphene in large sheets using methane CH4 as carbon source, but the process also requires high temperatures. Plasma (electrically ionized gases) CVD reduces the temperature required, but involves expensive equipment and producing multilayer graphene with low transparency and many defects resulting from ion bombardment by the plasma.
Researchers at the National Central University of Taiwan led by Chien-Cheng Kuo have devised a low-cost plasma-assisted CVD to grow monolayer graphene at low temperature and without the defects from the ion bombardment [4]. The plasma is generated from the gases in a separate chamber between two electrodes, which then flows into the chamber where deposition occurs onto copper foil (see Figure 1). The flow rate of H2 was varied from 5 to 20 standard cubic centimetres (sccm) per second at a temperature as low as 600 ºC. A direct current pulse power supply of 200 W with pulsing frequency of 20 kHz maintains the plasma. A spectrum analyser was used to obtain the plasma emission spectra through an optical fibre.
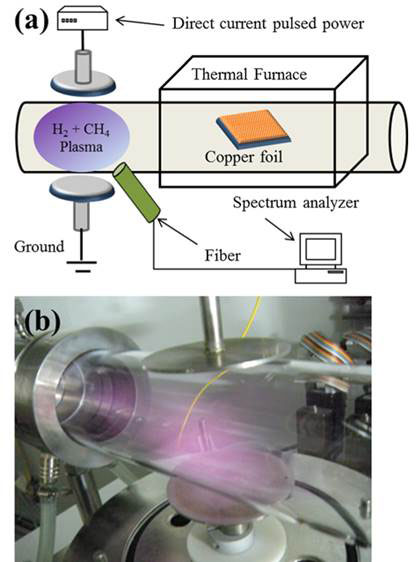
Figure 1 Plasma assisted CVD at low temperature for graphene synthesis
Graphene films were grown on 25 mm thick copper foil electro-polished and washed, then mounted in the CVD chamber and the furnace heated to 1 035 ºC to anneal the sample for 30 min. Graphene was grown at a lower temperature of 600 ºC. Methane gas flowing at 1 sccm was the carbon source. It was mixed with various flows of H2 and fed into the tube for 5 min to form a monolayer of graphene (Figure 2).
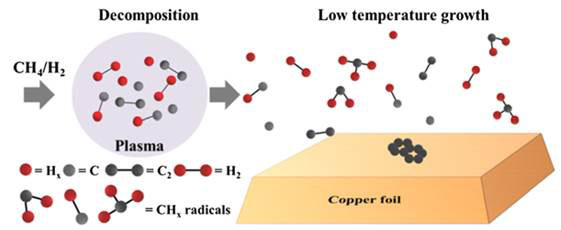
Figure 2 Plasma decomposed fragments for CVD graphene synthesis
Graphene growth involves the decomposition of CH4 and H2 in the mixed plasma into fragments including CHx radicals (chemical species with unpaired electrons). The gaseous CHx radicals recombine with each other after they had floated for a certain distance, and the metastable carbon atoms and molecules form graphene structure on the copper surface. Crucially, the most effective length for growing graphene between the plasma and the centre of the hot zone was approximately 30 cm.
Subsequently, the sample was rapidly cooled by removing it from the hot zone of the furnace. The synthesized graphene films were transferred onto SiO2/Si substrates by etching away the copper foil in an ion chloride solution. Prior to wet etching, a 200-nm thick film of PMMA (poly-methyl methacrylate) was spin-coated onto the top of graphene/copperfoil and baking at 13o ºC for 1 min. The PMMA/graphene thin films were washed with dilute hydrochloric acid to remove the metal ions and then rinsed deionized water. PMMA/graphene films were placed on the SiO2/Si substrate and the PMMA was dissolved away in an acetone bath over 24h.
Spectroscopic analysis showed that higher quality graphene was obtained with increased H2 flow rate.
Another group at Chinese Academy of Sciences in Shanghai, China, led by Li Tie and Wang Yue-Lin found that graphene domains can be better synthesized on electroplated Cu with a smooth and uniform surface from dilute methane gas [5]. The copper electroplating was performed on a SiO2/Si substrate after 50 nm ni was sputtered on for the adhesion layer, and then 200 nm Cu sputtered on as the seed layer. Electrodeposition was finished in a plating tank with copper sulphate to about 4mm. Graphene synthesis was done in a tube furnace with a mixture of CH4 (0.15 sccm), H2 (50 sccm) and Ar (500 sccm) at ambient pressure and 1 040 ºC for 3 to 10 minutes.
Hexagonal domains of graphene were deposited increasing in size with increasing length of deposition time ranging from 2 up to 7 mm in diameter. These were far superior to those grown on copper foil: denser coverage and larger domains. Further work indicates that the graphene nucleation density was proportional to the CH4 concentration kand the graphene coverage rate on electroplated Cu was proportional to the CH4 concentration and growth time.
There is clearly much room for improving the efficiency and cost-effectiveness of the process, as well as the quality of the product.
Carbon dioxide, the major greenhouse gas responsible for global warming could be reduced converting it into liquid fuels, useful chemicals or carbon materials. Currently attractive options for transformation of carbon include reducing CO2 to diamonds and nanotubes, either through direct CO2 splitting or by reacting with metals at pressures > 70 MPa (~ 700 atm).
Scientists at City College of New York in the United States have succeeded in converting CO2 to graphene oxide using relatively non-toxic materials and mild conditions [6]. The conversion takes place in two steps: CO2 fixation (at CO2 pressure < 3 MPa and temperature < 100 ºC) and graphenization (600 -75o C under 0.1 MPa of N2). The first step generates a solid that contains methoxy (OCH3), formate (HCOO) and aliphatic (C-C) groups while the second step pyrolysis the solid compound to graphene oxide-boron-oxide nanocomposites. The formation of aliphatic groups without using metal-containing compounds at mild conditions is of great interest to the synthesis of various organic products starting from CO2.
An extremely simple method of arc discharge shows promise in producing graphene sheets with a few layers. An arc discharge is simply a bright electric current that forms across a gap in a circuit or between two electrodes. Researchers led by Yongsheng Chen at Nankai University, Tianjin, China, used a direct current arc-discharge in a home-made water-cooled stainless steel chamber filled with a mixture of CO2 and helium [7]. Different gas compositions ranging from 5 vol% to 40 vol% CO2, and direct currents of 100 to 200 A were used. The discharge voltage was kept around 30 V by controlling the distance between the two electrodes. Both electrodes were normal graphite rods obtained commercially with an anode diameter of 13 mm and a cathode diameter of 40 mm. After discharge, the cotton-like deposits that forms on the inner wall of the chamber was collected and examined. The optimum conditions for producing a few layered graphene were a low voltage < 35 V, high gas pressure (>1 270 Torr ~1,7 atm), high current (~150 A) and 25-40 vol% CO2. In a typical run, tens of grams of high-quality graphene sheets with four to five layers can be generated in minutes.
Researchers led by Narayan Hosmane at North Illinois University DeKalb in the United States discovered a quick method for converting carbon dioxide directly into graphene simply by burning the metal magnesium in dry ice according to the reaction [8]:
2Mg + CO2 → 2MgO + C
In a typical experiment, 3 g of Mg ribbon was ignited inside a dry ice bowl and covered by another dry ice slab. After combustion, the black products were transferred to a beaker containing 100 mL of 1M HCl and stirred overnight at room temperature to remove Mg and MgO as MgCl2, which is soluble. After washing, the isolated solid carbon product was dried under high vacuum overnight at 100 ºC, yielding 680 mg (92 % of theoretical). The product was characterized by TEM, Raman spectroscopy, wnergy-dispersive X-ray analysis and X-ray powder diffraction, and found to be 3-7 layers graphene, along with trace amounts of Mg and O.
A company Graphene Technologies has patented a technology to produce graphene from CO2, and was named Company of the year in 2013 by the Futures in Review conference.
Article first published 07/08/13
Comments are now closed for this article
There are 2 comments on this article.
Todd Millions Comment left 25th August 2013 18:06:18
Since you mentioned diamonds,back in the pre interweb days(bless the sainted Al Gore.),Diamond films where reported grown on substrats,covered with diamond dust 'seed'.In methane filled chambers,by using a laser to catalysis the film growth.I don't recall the temps,pressures or wavelenghts.The source was popular science-I think.I never got the scratch proof lexan windsheild,glasses or unbreakable solar panel glazing I expected from this-fart to diamond trick-but graphene 'seed',and CO2 for same approach may be worth persuing.Cheers.
Philip Ward Comment left 20th October 2013 22:10:59
Before you claim such a tehcno-fix can "save the climate", isn't an audit of embodied energy in the product and a discussion of issues of scale is necessary?