Water bound on surfaces of proteins and membranes conducts positive electricity, and could enable cells and tissues to intercommunicate rapidly and efficiently. Dr. Mae-Wan Ho
For decades, scientists have wondered whether water bound to the vast amounts of surfaces of proteins and membranes inside the cells could conduct electric charge in a very special way. If the water molecules were aligned with their positive and negative charges alternating in a chain, as would be the case if adjacent water molecules were linked together by hydrogen-bonds (a kind of chemical bond involving a hydrogen being shared between two oxygen atoms), then a ‘jump’ conduction of positive electricity could, in theory, take place. This involves the positive charge of the hydrogen nucleus - a proton – passing rapidly down the chain by relay, without the proton actually moving down. The free proton takes over bonding with the oxygen of the first water molecule in the chain, creating a second free proton that displaces its neighbour down the chain until the last proton comes off at the other end [1] (Fig. 1). Jump conduction is faster than ordinary electricity passing through a metal wire, which involves electrons actually moving, and much, much faster than conduction by charged ions diffusing through water. But it needs to have chains of water in a sufficiently ordered state and protein and membrane surfaces may impose that kind of order on water.
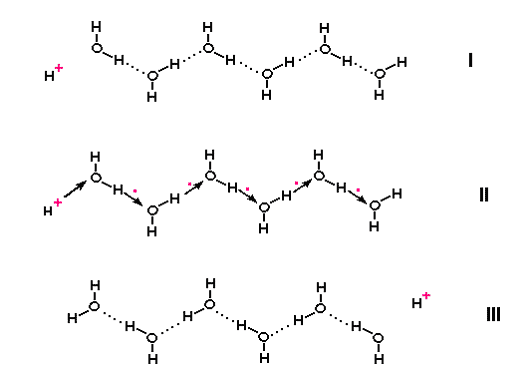
Figure 1. Jump conduction of protons along a chain of water molecules.
Within the past 10 years, evidence for jump conduction of protons via daisy chains of water molecules has come from several sources.
According to the story in biochemistry textbooks (and you need a good one to even tell you that), living organisms are charged up predominantly by accumulating protons on one side of a membrane, and discharged by protons flowing back down to the other side. Protons are transported across biological membranes by special membrane proteins called “proton pumps”. The protons pumped uphill (to a higher energy state), using an external energy source, such as the oxidation of foodstuff, or absorption of sunlight, is returned downhill via another enzyme, ATP synthase embedded in the same membrane, which uses the energy to make ATP, the universal energy intermediate that powers all living activities. This “chemi-osmotic hypothesis” won a Nobel Prize for British biochemist Peter Mitchell who first proposed it. The protons are supposed to exist in bulk solution on either side of the membrane, and it is the difference in concentration between the two compartments separated by the membrane that drives the synthesis of ATP.
Structural studies carried out on these proton pumps within the past ten years show that they form a channel through the cell membrane that is threaded by a chain of hydrogen-bonding water molecules from one side of the membrane to the other [2]. Examples of these proteins are the bacteriorhodopsin, the light-harvesting pigment of the purple membrane belonging to a bacterium, and the cytochrome c oxidase that catalyses the last stage in the oxidation of foodstuffs in the membrane of the mitochondria (the powerhouses of the cell), in which oxygen is reduced to water by combining with protons and electrons.
However, biochemists have noticed that the rate of some proton pumps, such as the cytochrome c oxidase - which pumps more than 103 protons per second - is higher than the rate at which protons can be supplied to the proton conducting channel via the bulk diffusion rate [3]. And since the chemiosmotic hypothesis was first proposed, it has been suggested by chemist R.J.P. Williams in Oxford University [4], and others subsequently [5], that the protons, rather than accumulating in solution in the bulk of the cell compartment, actually diffuse along the membrane surface; perhaps directly from proton pumps such as cytochrome c oxidase enzyme to the ATP synthase embedded in the same membrane.
Experimental observations have suggested that proton conduction could indeed take place along the surface of both natural and artificial membranes at the interface with water, and more specifically in the water layer(s) immediately next to the membrane surface [6]. The long-distance migration of protons along membranes has been observed in purple membranes and reconstituted bacteriorhodopsin, which demonstrated a high rate of diffusion of protons along the membrane surface and a tendency for protons to remain on the membrane surface as opposed to going into the bulk of the cell compartment.
When protons diffuse along the surface of membranes instead of through the bulk solution, the rates of proton transport processes are significantly increased [3]. This is due to a fundamental difference of diffusion in two as opposed to three dimensions. In three dimensions, a proton far away from its target - say, the entrance to a proton pump embedded in the membrane - will have a very small probability to be caught by the target. But in two dimensions, the probability of the proton being caught is exactly 1; in other words, it will be caught sooner or later. And if instead of random diffusion, protons are jump-conducted along chains of interfacial water molecules aligned along the membrane surfaces, then proton transport processes can indeed be quite fast.
Researchers in the Max-Planck Institute of Biochemistry, Martinsried, Germany first showed that very thin films of water (down to about one layer) adsorbed onto a solid surface exhibits a “surprisingly high conductivity” while using a scanning tunnelling microscope [7]. The scanning tunnelling microscope depends on the flow of an electrical current and thus cannot be used to directly image insulating material. But in humid air, a thin film of water settles on the surface, and is sufficient to provide sufficient electrical conductivity to allow imaging at currents below 1 picoampere.
A model of proton-conducting water chain or “proton-wire” has come from a further unexpected source: studies on carbon nanotubes. A carbon nanotube is a new form of carbon discovered in 1991 in which carbon atoms are joined up into the shape of a long thin tube. Such tubes are typically of nanometre diameter, and could be microns in length. These nanotubes are found to interact substantially with water.
Scientists from the National Institutes of Health, Maryland, and the University of Maine in the United States simulated experimental results on the computer [8]. They showed that a single-wall nanotube 1.34 nm long and .81nm in diameter rapidly filled up with water from the surrounding reservoir, and remained occupied by a chain of about 5 water molecules on average during the entire 66ns of simulation (a nanosecond is a billionth of a second, or 10-9s, which is a long time in the life of a molecule).
This result was surprising, because carbon does not have a high affinity for water. But it seems that getting into tight places restricts the distribution of energies in the water molecules, so they end up with a lower average energy than if they were in bulk water, and hence it becomes energetically favourable for the water to enter the nanotubes.
An analogy I can offer is how, in a crowded underground carriage, people’s movements are restricted, and hence the range of energy distribution is narrowed towards the lower end of the scale.
Hydrogen bonds between water molecules inside the nanotube are shielded from fluctuations in the environment, and are much more stable. Within the nanotube, only 0.02 percent of pairs of water molecules in contact distance (0.35nm) are unbound, compared with 15 percent in bulk water. H-bonds in the nanotube are highly oriented, with less than 15 percent of the H-O….O angles between adjacent water molecules exceeding 30o, compared to 37 percent in bulk water. The average lifetime of a H-bond inside the nanotube is 5.6 ps (picosecond, or10-12s), compared to 1 ps in bulk water. The H-bonds are nearly aligned with the nanotube axis, collectively flipping direction from one side to the other every 2-3 ns on average.
Water molecules not only penetrate into the nanotubes, but are also conducted through them. During the 66 ns, 1 119 molecules of water entered the nanotube on one side and left on the other, about 17 molecules per ns. This rate is comparable to that measured through the twice as long channel of the transmembrane water-conducting protein, aquaporin-1. Water-conduction occurs in pulses, peaking at about 30 molecules per ns, again reminiscent of single ion channel activity in the cell; and is a consequence of the tight H-bond inside the tube.
There is a weak attractive force between the water molecules and the carbon atoms, (‘van der Waals force’) which is 0.114 kcal per mol. Reducing this by 0.05 kcal per mol (less than 5 percent) turns out to drastically change the number of water molecules inside the nanotube, which fluctuates in sharp transitions between empty states (zero water molecule) and filled states, suggesting that changes in the conformation (shape) of enzyme protein molecules may control the transport of water from one side to another in the cell membrane.
Do such water-filled channels conduct protons? The answer is yes. If there is an excess of protons on one side of the channel, positive electricity will spirit down fast, in less than a picosecond, some 40 times faster than similar conduction of protons in bulk water, according to Gerhard Hummer of the National Institutes of Health in the United States, the leader of the team that carried out the nanotube simulation studies [9].
If the nanotubes, instead of swimming in free water solution, were immobilised in membranes, they could be used for all kinds of applications, including light sensing, field effect transistors for proton currents, and desalination of seawater.
What role does interfacial water play in the life of an organism? Everything, it seems (See previous “New age of water” series, SiS 23, SiS 24). Interfacial water accounts for some 70 percent by weight of most organisms including human beings, making organisms effectively liquid crystalline. I have proposed some years ago [5] that proton-conduction through interfacial water may be how the body intercommunicates at all levels, enabling it to function as a perfectly coordinated whole. This idea is gaining ground [10] (See “The liquid crystalline organism and biological water” https://www.i-sis.org.uk/onlinestore/papers1.php#section3).
Article first published 27/10/05
Comments are now closed for this article