China's transgenic maize with high phytase to make phosphate available has had its bio-safety certificate renewed without requisite molecular characterization and supporting safety data; it is a poor strategy for overcoming phosphorus scarcity, and has the potential to cause serious harm to health and the environment Dr. Mae-Wan Ho
China’s first transgenic maize with greatly enhanced levels of phytase was created in 2008 by academician Yun-liu Fan and her team at Biotechnology Research Institute, Chinese Academy of Agricultural Science in Beijing [1]. It was granted a ‘bio-safety certificate’ in 2009 for 5 years, which failed to be renewed in August 2014 when it expired [2]; and it seemed to the outside world that China had given up on its genetically modified (GM) rice and corn on account of strong consumer rejection. However, at the beginning of 2015, the Chinese company Origin Agritech Limited based in Beijing’s Life Science Park announced that the bio-safety certificate for the transgenic phytase maize has been renewed [3].
Origin Agritech Limited describes itself as [4] “China’s leading agricultural biotechnology company specializing in crop seed breeding and genetic improvement, seed production, processing, distribution and related technical services. As the first Chinese seed company with an in-house biotech research center, Origin leads the development of Genetically Modified (GM) technology.”
In addition to the original phytase maize, the company has further incorporated phytase traits into two of its best-selling commercial corn hybrids; and commercialization of these two corn hybrids is pending approval from the Chinese government. They have also transformed herbicide resistance, insect resistance and drought stress genes into corn inbred lines. However, the company’s portfolio includes many conventionally bred crop varieties and marker assisted breeding is done to improve corn and rice.
Phytase is an enzyme that breaks down phytic acid (Figure 1), an inositol (a carbohydrate) with 6 phosphate groups, also known as inositol hexkisphosphate [5].
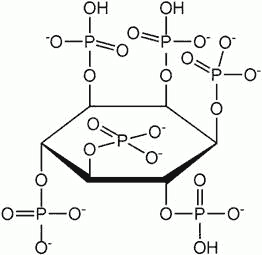
Figure 1 Phytic acid (also known as inositol hexakisphosphate)
In seeds, phytic acid is deposited as a mixed salt within protein storage vacuoles. Besides sequestering inorganic phosphate, it also binds divalent cations such as Fe2+, Mn2+, Mg2+, Zn2+ and Ca2+. Early research had suggested that phytic acid is an anti-nutrient, because it interferes with the utilization of inorganic phosphate as well as the minerals chelated by phytic acid. Proponents suggest that reducing the phytic acid content of grains would be beneficial for human health [6]. In the developing world, the high phytate in grains can contribute to iron and zinc deficiency if the diet is poor and deficient in minerals. In the developed world, low phytate in grains could have nutritional and environmental benefits for animal agriculture. While ruminants normally have microorganisms in the gut that digest phytate, monogastric animals such as pigs, poultry and fish do not. They produce manure high in phytate phosphate that’s unavailable to crops and instead pollutes water, resulting in eutrophication. Farmers have to supplement animal feed either with rock phosphate, a non-renewable resource getting increasingly scarce (see [7] Phosphorus Starvation Threatens the World, SiS 61), or add phytase isolated from bacteria to release phosphate from grains after ingestion.
Beginning in the early 1990s, several low phytic acid (lpa) mutants were isolated in maize, barley, rice, wheat, soybean as well as Arabidopsis thaliana (reviewed in [6]). Plants homozygous for the lpa typically produce seeds in which phytic acid is reduced by 50 to 95 %, and almost always with corresponding increase in available phosphate. Animal feeding studies confirmed that lpa seeds provide more available phosphorus and reduce phosphorus in animals waste.
The problem with systemic reductions of phytic acid levels in the mutants is that it often has negative impacts on seed and plant performance, such as reduced germination, emergence, stress tolerance and seed filling. Genetic modification offers a more targeted approach that can disrupt phytic acid accumulation in seeds without reducing seed dry weight or impairing germination.
Yun-liu Fan and colleagues created transgenic maize plants expressing high levels of phytase gene (phy A2) from the fungus Aspergillus niger in the seeds, using a construct driven by the maize embryo-specific globulin-1-promoter [1]. This limits the disruption of phytic acid accumulation to the seed without affecting other vital functions of the plant.
The phyA2 gene from A. niger line 963 encodes 57 amino acids with a signal peptide at the N terminus for extracellular secretion. The yeast-expressed recombinant phyA2 enzyme lacks the signal peptide, and remains intracellular. It has high specific activity on phytic acid with two optimum at pH 1.6-2.0 and 5.5-5.9. The specific actvity at pH 1.8 is 77 % that at pH 5.8. At pH 3.0 - average pH in animal digestive tract - phyA2 retains 40 % of activity. The widely used phyA enzyme has only 25 % activity at pH 3.0. The phyA2 enzyme is marketed as commercial feed additive in China.
Two vectors were constructed with the maize embryo-specific globulin-1 promoter and terminator for the A. niger phyA2 gene. One of them has a signal peptide sequence from the barley a-amylase gene. In addition, a plasmid carrying the maize histone H2B promoter, the maize ubiquitin 5’UTR intron-1, the bar gene (for glufosinate resistance) and the potato protease II terminator was used as the selectable marker.
The phytase expression cassette was excised with restriction enzyme for transformation, as was the bar gene expression cassette. Transformation was carried out with tungsten microprojectiles carrying the expression cassettes.
Thus, the transgenic phytase maize is created not by inserting a gene precisely at a chosen site in the genome; but by shooting in a mixture of two different gene expression cassette, one for the phytase, and the other for the selectable marker, each made by joining together several different sequences, and hoping that the two cassettes land together somewhere, and in some form, in the genome. In other words, there is no control over where and in what form the foreign DNA sequences land in the genome; furthermore, there will be many collateral damages to the genome caused by the process. All that makes for unpredictable effects with regard to bio-safety.
Basta (Bayer, AG) paste was used for a leaf painting assay to identify transformed plants carrying the bar gene at the five-leaf stage.
A total of 40 independent transgenic events were obtained; 33 without the signal peptide sequence and 7 with the sequence. All events produced T1 seeds. T1 seeds were produced by crossing the T0 seeds with a non-transgenic line. Phytase activity was determined using 5 randomly selected seeds. Based on the phytase activity in T1 seeds, selected events were planted in the greenhouse and self-pollinated to produce T2 seeds. Two events were propagated to produce T4 and T5 seeds. The germination frequency ranged from 75 to 88 % in the field and 80-92 % in the greenhouse, the phenotypically ‘wild-type’ plants (with low phytase activity) behaved similarly. The T2 segregation was consistent with (but does not prove) Mendelian inheritance for a single locus (site) for bar gene, and 16 out of 20 bar plants showed phyA2 gene on PCR analysis.
Southern blot analysis was carried out to estimate copy number of the transgene in the event selected, and interpreted as possessing two copies of the transgene. The phyA2 protein expressed in yeast is glycosylated (with carbohydrate chains added) and has a molecular weight ~75 kD; when de-glycosylated, the protein has a molecular weight ~55 kD. The transgenic phyA2 protein was ~60 kD, indicating it is glycosylated differently from the yeast protein. The T4 seeds were assayed for phytase activity and had 2 200U/kg on average, about 50 times that of the wild-type control. The phytic acid of wild-type control seeds ~3.3mg/g was reduced o 2.39-2.66 mg/g in transgenic seeds. The Pi (inorganic phosphate) contents were ~0.12 and 0.41-0.56 mg/g in wild-type and transgenic seeds respectively. Thus, phytic acid was reduced by 23% and Pi increased by 3-fold.
The paper describing the creation of the transgenic phytase maize was accepted in August 2007, and published in 2008. By 2009, one of the events had acquired a bio-safety certificate.
Yet, there has been no molecular characterization of the transgenic phytase maize event, no sequencing, mapping, no profiling of nucleic acids, proteins, or metabolites compared to the isogenic parental line. This is unacceptable at a time when these methods are routine, and there is general acceptance that genetic modification results in uncontrollable, unpredictable changes in RNA transcripts (including numerous small RNA species with regulatory functions), proteins, and metabolites that can impact on safety (see [8]). Although the glycosylation appeared to be different in the transgene compared with the yeast enzyme, no further analysis was carried out, nor any studies of allergenic potential in vivo, in vitro, or in silico. There is evidence that a change in glycosylation in a transgenic protein can give rise to serious immunological reactions in an otherwise harmless protein [9]. There have been no molecular genetic data documenting stability of transgene inserts in successive generations of being grown in the field. That is most important, as genetic instability will nullify all safety tests that have been carried out (see [8]). The consistency with Mendelian ratios in T2 segregation [1] was the only observation from which a single locus was inferred. Not only is that a weak test for single locus; it is not evidence for genetic stability without molecular characterization.
There have been a few short-term and very limited studies on health and environmental impact.
A feeding trial lasting 16 weeks on 50-week old laying hens with 62.4 % transgenic phytase maize in the diet compared with the same percentage of conventional nontransgenic maize found no difference in organ weight relative to body weight, or in a number of serum biochemical parameters, or the digestibility of dry matter, energy, nitrogen and calcium [10]. Neither the maize-specific invertase gene (ivr) nor the transgene (phyA2) were detected by PCR in the breast muscle, leg muscle, ovary, oviduct and eggs. But no information was given as to the limit of detection for the method used. The only difference detected was the digestibility of phosphorus: 58.03 % in transgenic maize-fed hens vs 47.42 % in controls. The observations did not include body weights of the hens, the number of eggs laid, or any other indications of well-being. Also, old adult hens are not the best experimental animals for detecting adverse responses to transgenic high phytase maize in the diet.
In another feeding trial lasting 12 weeks, 44 week old brown laying hens were tested for their ability to use phosphate partially provided by the transgenic phytase maize (360 Units/kg diet, equivalent to only 1.7 % of transgenic phytase maize) in combination with non-phytate phosphate (NPP, 0.26 %, 0.21 % or 0.16 %) compared with hens provided only with 0.26 % NPP or 0.36 % NPP [11]. Not surprisingly, there was no significant difference in egg production, average daily feed intake, feed efficiency, rate of broken or soft-shelled egg production or egg mass among the treatments, except that the hens given only 0.36 % NPP produced more phosphate in their faeces.
Two herbivore species Otrinia furnacalis (maize borer) and Helicoverpa armigera (corn earworm), both serious pests of maize crops, were tested for their survival on transgenic phytase maize kernels compared with near-isoline nontransgenic kernels [12]. The results showed no difference in survival and duration of the first and second instars and fresh weight of the third instar. The same results were obtained with maize meal instead of whole kernels.
However, another team of researchers found that transgenic phytase maize significantly enhanced pupal weight and female fecundity of the maize borer, and the length of the maize borer feeding tunnels in the stem significantly greater in the phytase maize. Moreover, there were significant increases in adult weight and population abundance of the maize weevil. Altogether, the increased insect-induced loss was ~8 % in the transgenic maize compared with the non-transgenic maize [13].
A few field monitoring experiments were carried out for one season only. No significant adverse effects of transgenic phytase maize were found, or recognized, for the biodiversity of carabid beetles [14], or arthropods [15], or soil nematodes [16].
As Prof Joe Cummins stated in his article [7], high phytase transgenic crops “do not have the ability to fabricate phosphorus to replace that required for human nutrition in the long run.” Nor do they recover phosphate that has been lost in wastes and run0ffs that pollute water ways. Major remedies for phosphate depletion involve phosphorus recycling, such as recovery from municipal waste, from animal bone, and recovery through green manure with long roots that can extract phosphate from deep soil layers.
But there are other problems with potential health impacts of high phytase grain. No long- term feeding trials have been carried out on any animal, and none whatsoever on livestock, short- or long-term, for which the grain is intended. The potential impact on human health could be substantial, considering that, as acknowledged by a proponent of low phytic acid transgenic strategy [6]: “Phytic acid is ubiquitous in eukaryotes and regulates many cellular functions, including stress responses, development, phosphate sensing and homeostasis, DNA repair, RNA editing and mRNA export..”
Furthermore, the suggestion that reducing phytic acid content of grains could have significant beneficial effects on human health in the developing world and environmental and animal health benefits for livestock agriculture in the developed world [6, 1] (see above), have been strongly contested. The evidence for both was described by a critic as “flimsy at best” [17]. Recent studies show that the ‘anti-nutrient’ effect of phytate is manifest only when large quantities of phytate are consumed in combination with a diet poor in trace elements. Besides, the mere addition of citric acid to feed has been shown to increase phytate-phosphorus use [18]. New Hampshire x Columbian crossbred male chicks and commercial broiler male chicks from 8 to 22 d of age were fed ordinary corn with varying amounts of citric acid. Citric acid was found to improve phosphorus utilization and weight gain as well as gain/feed ratio and bone ash (of tibia). The results suggested that 3 to 4 % citric acid can release or spare between 0.05 and 0.1 % P. The mechanism of citric acid action is still unclear; the authors speculated that citric acid could chelate Ca to prevent the formation of insoluble Ca phytate complex.
In fact, phytate itself [17] has been consistently and reproducibly associated with health benefits, including broad-spectrum anticancer activity [19], enhancement of natural killer cell activity, and prevention of kidney stones and calcification.
Animals including humans have very low levels of phytase, though rats have 30 times that of humans (see [20] and references therein). In general humans do not produce enough phytase to safely consume large quantities of high phytate food on a regular basis. But probiotic lactobacilli and other species of endogenous digestive microflora can produce phytase, even in monogastric animals.
Unfortunately, these bacteria are susceptible to glyphosate herbicides, increasingly large quantities of which have been used on our farms, gardens, parks, residential and commercial areas based on false claims perpetrated by Monsanto with the collusion of regulators that glyphosate is harmless to human beings even in the face of overwhelming evidence to the contrary (see [21] A Roundup of Roundup Reveals Converging Pattern of Toxicity from Farm to Clinic to Laboratory Studies, SiS 65).
As Anthony Samsel and
Stephanie Seneff wrote in their review on glyphosate [22]: “Lactobacilli and
other beneficial gut bacteria produce the enzyme phytase, which catalyses the
release of phosphate from phytates and improves the intestinal absorption of
important minerals such as iron and zinc... Because glyphosate reduces the
number of these types of bacteria in the gut, it should enhance the chelating
potential of phytates. This is likely a protective measure to avoid excess
bioavailability of free phosphate, which is problematic in transport in the
presence of glyphosate. Glyphosate’s known ability to itself chelate
divalent cations is likely a factor as well [in zinc deficiency]. Zinc
deficiency increases
the risk of diarrhea, pneumonia and malaria in infants and young children.”
The use of glyphosate not only kills off beneficial bacteria in the gut of human beings, but also in the gut of animals including ruminants, which suffer severe diarrhoea from Clostridium as a result [23].
In other words, the way to improve animal nutrition and human health is not through transgenic high phytase maize, but pesticide-free organic agriculture.
High phytase maize, if excreted along with animal manure could itself cause problems with soil phosphate content and phosphate leaching from the soil resulting in eutrophication. This comes from experiences of farmers on the ground. Howard Vlieger, a farming consultant based in Iowa tells me [24]: “There is significant reason to believe that the phytase used in livestock production in the US could be mobilizing phosphorus from the soil. There are a growing number of instances where the phytase-treated manure is applied to crop land and the phosphorus levels are declining even with repeated applications of manure containing phosphorus.”
Arthur Dunham, Iowa-based clinical veterinarian give further details [25]. He tells me that industry started adding phytases to poultry and pig diets in the 1990s; Nutraphos sold by BASF was one of the leading products. It contains phytase from E. coli and the feed industry hated working with it because it was a small unstable protein that could not withstand the heat of pelleting, so the phytase had to be sprayed on after pelleting. BASF was the first to find some E. coli strains that produced heat-stable phytase so they switched to them about 2006, and the rest of the industry followed suit, as all ingredients could be put in the premixes ahead of pelleting.
The increased stability of the phytase is the problem. “No one thought about what might happen if this product which breaks down phytate to make P more available keeps working in the pit and out in the field.” Arthur says.“Phytate is the part of organic matter that holds inorganic phosphate, and keeps it from leaching away like nitrate, unless there is soil loss. With the use of the new stable phytases, the available phosphorus in soil can drop like a rock. A slight spring rain or snow melt can carry the phosphate away, or the phosphate is just running off with nitrate in the tile water.”
Worse yet, this phosphate also takes the place of the glyphosate bound to Ca2+ and Mg2+ so it can free up the glyphosate to be an active antibiotic again, Arthur suggests, along with Dr. Mike McNeill, an agronomist at Iowa State University. “We have some soil test data and some clinical data,” Arthur says, “but we are not getting much support.” A few swine producers are adding a product called Accomplish with their starter fertilizer which is a phytase from Syngenta to try to correct the crash in P for their crop! “If I could consistently find some of the original less stable phytase products, I would approve of my swine clients using them.” Arthur adds.
“How do we know how stable the phytase produced in this GMO corn is going to be and how are we going to keep a swine producer from trying it on top of the phytase in his feed? Dr. McNeill and I know of some swine clients that have seen available phosphorus levels go from around 60ppm to under 5ppm in a couple years. And it all goes to our lakes.”
The bio-safety certificate has been granted and renewed for transgenic phytase maize without proper risk assessment. Existing evidence strongly suggests that commercial release of the transgenic phytase maize could be disastrous for health, agronomy and the environment.
To safeguard public health and the environment, China’s Ministry of Agriculture, which has renewed the bio-safety certificate, should make all test reports submitted to the Ministry for the transgenic phytase maize available for open scientific review. More importantly, the Ministry should support independent long-term toxicological and environmental impact studies on the transgenic phytase maize (or hybrids containing the high phytase trait) before any commercial release is contemplated.
Article first published 17/06/15
Comments are now closed for this article
There are 1 comments on this article.
Todd Millions Comment left 28th June 2015 05:05:38
A trial of a whole 16 WEEKS , on already mature test animals!? Congratulations China. Ag/Tox and Health/Pharmawhore Canaduh will be green with envy when they learn of this testing 'efficiency '. If I might be a boor-given the record you have of some thousands of years(According too-'Farmers of 40 centuries')in the recycling of Fey sourced phosphates(and other needed minerals), pherhaps an examination of how much of these sources is ending up flushed to the oceans via the watercourses should be a slightly higher priority than this maize mod work. Recovery is easier before this point, as is siting application. Besides there is a great export potential for such methods to areas populated by ignorant monotheist barbarian peoples- Like the frosty banana republic I'm in and from.