Stable structures containing billions of water molecules in highly dilute solutions of chemical compounds are formed only in the presence of ambient electromagnetic fields; they exhibit physical properties distinct from bulk water and are essential for biological activity Dr. Mae-Wan Ho
Mention highly dilute solutions and homeopathy comes to mind along with the ferocious attacks and dismissal by the conventional scientific and medical community indoctrinated on a mechanistic biology that’s fast becoming obsolete. Within the past decade, new findings in the quantum physics and chemistry of water have put water at the centre stage of cell and organismic biology (see [1] Living Rainbow H2O, ISIS publication) based on a new framework of quantum electrodynamics field theory of condensed matter [2]).
Simultaneously, as Academician Alexander Konovalov at Arbuzov Institute of Organic and Physical Chemistry, Russian Academy of Science Kazan Science Center, Tatarstan, points out [3], thousands of papers have documented that solutions of biologically active substances in water can give biological effects not only at ordinary concentrations of 10-3 to 10-7 M, but also at very high (homeopathic) dilutions, separated by concentrations in between that have little or no effect. This U-shaped curve is so prevalent that it has been given a name: ‘hormesis’.
Over the past 6 years, Konovalov and his team have studied about 100 compounds at 10-2 to 10-20 M, diluted sequentially with rigorous shaking (succussion) starting from the initial solution (reviewed in [3, 4]). The list includes antioxidants, plant growth regulators, neuro-mediators, vitamins, tranquilizers, hormones, various drugs as well as substances of unknown biological effects. The compounds range from simple molecules like glycine to complex macrocyclic compounds like porphyrins or calyxarenes.
They monitored electric conductivity, surface tension, pH and in some cases dielectric permeability and optical activity at different dilutions. To measure the size of nanostructures formed in solution, the team used dynamic light scattering (DLS). DLS is a physical technique generally used for determining the size distribution of small particles in suspension or polymers in solution [5]. Water chemists have discovered that it also enables the detection of nano-objects in highly dilute solutions that have very few solute molecules left, and this has greatly facilitated research on such solutions. At the same time, the technique determines the surface electrical (zeta) potential of the nano-objects.
Recently, the experiments were carried out both on the lab bench and within a three-layer permalloy (iron/nickel) container shielding out external electromagnetic fields. For example, the geomagnetic field was brought down to a thousandth of its normal level.
A quarter of solutions behaved ‘classically’, i.e., highly dilute solutions become like bulk distilled deionized water in surface tension and electrical conductivity; but the majority, 75 % behaved non-classically, as for example, the antioxidants phenozane and a-tocopherol (vitamin E).
Phenozane on being diluted in aqueous solution exhibits a fall in surface tension by 10-20 mN/m at around 10-6 to 10-7M, while electrical conductivity rises to 40 mS/cm (S, Siemen is 1 Ampere/Volt) and keeps changing with subsequent dilution. These changes are accompanied by biological effects that vary significantly with dilution (Figure 1).
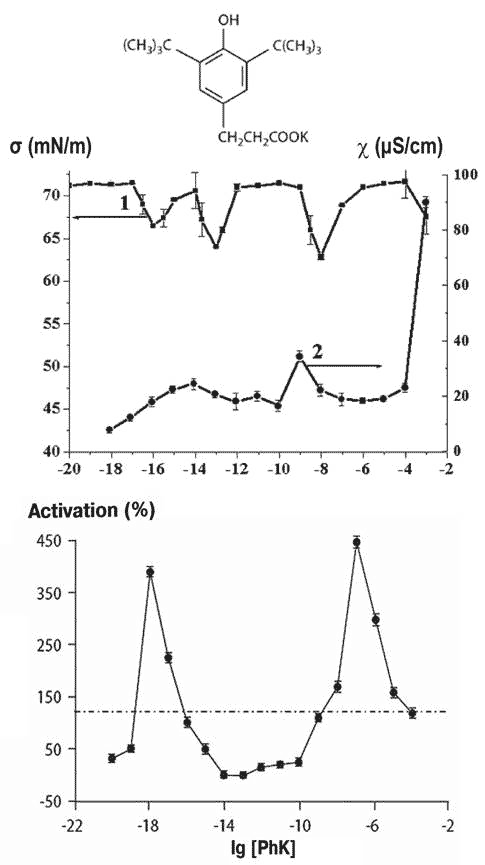
Figure 1 Solutions of potassium phenozane (top), and variation in the surface tension and conductivity (middle) and protein kinase C activation of cultured rat smooth muscle cells (bottom) as a function of dilution
At high dilutions of non-classical compounds, nanosize structures appear. Their dimensions change in subsequent dilutions – not in linear or monotonic fashion, but rather in jumps, and the formation of these nanostructures appear to be necessary for biological effects.
Samples kept in hypo-electromagnetic environments notably fail to form nanostructures below a certain dilution, the physical changes such as conductivity are absent (see Figure 2), and the shielded solutions were also without biological effects at these high dilutions.
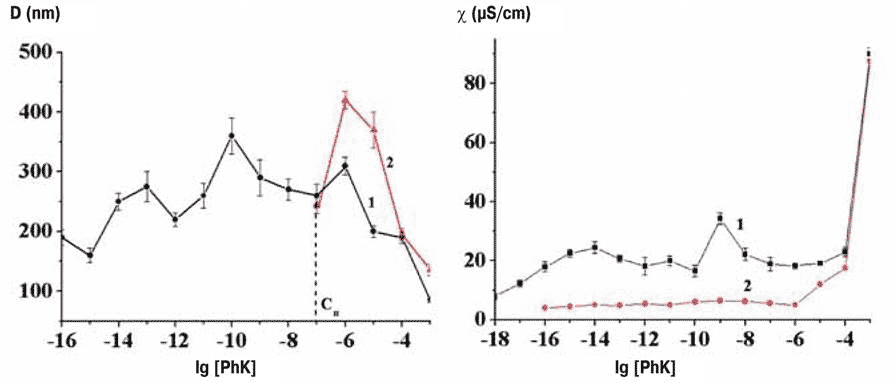
Figure 2 Formation of nanostructures on the lab bench (black line) and in permalloy-shielded environment (red line); left, dimensions of nanoassociates, right, conductivity
There appears to be a ‘boundary concentration, different for different compounds in the 10-5 to 10-8 range, beyond which nanostructures do not form inside permalloy containers, and where further biological effects normally show up.
A team at Emanuel Institute of Biochemical Physics carried out experiments to check on the hypothesis that biological effects are absent in the absence of nanostructures in highly diluted solutions. They looked at changes in the microviscosity of membranes exposed to potassium phenozane solutions. They found effects at 10-6 M corresponding to the usual maximum plus further peaks at 10-12 and 10-15 M for the dilute solutions kept on the lab bench; but the two additional peaks disappeared in solutions kept within the permalloy container (Figure 3).
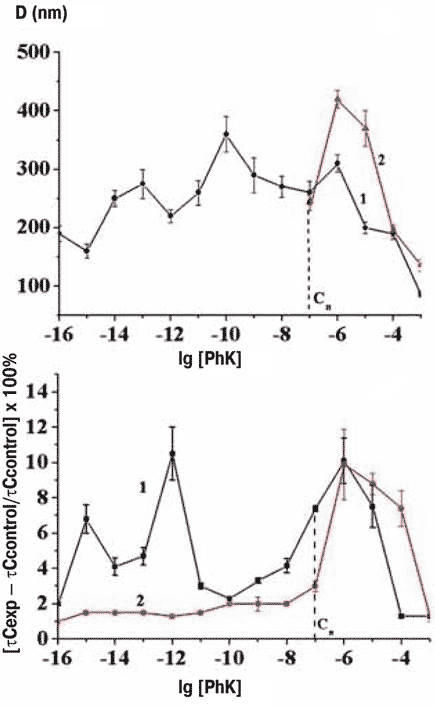
Figure 3 Formation of nanoassociates (top) and membrane microviscosity (bottom) in normal (black) and shielded (red) environments
As a further proof of concept, M. Sc. student Dmitry Konovalov experimented with solutions of cytyltrimethylammonium bromide. Under ordinary conditions on the lab bench, 240 nm nanostructures appear at 10-9M; this does not take place within the permalloy container. However, if a 7 Hz field is generated within the container, the nanostructures form to about the same size as on the lab bench [3].
The nanostructures are not due to nanobubbles or gases present in solution, as many believe. Using atomic force microscope in the semi-contact mode, Konovalov and his team successfully imaged the nanostructures (see Figure 4). At the lowest dilution (10-6M), spherical or semispherical particles are seen (most probably containing solutes) [4]. At higher dilutions, however, nanostructures are detected in the semi-contact mode, which have the characteristics of soft matter; and quite similar to those first identified and imaged on AFM by Shui-Yin Lo and his team at Quantum Health Research Institute Pasadena, California, in the United States (see [6] Large Structured Water Clusters Caught on Camera, SiS 61).

Figure 4 AFM images of nanostructures from solutions of amphiphilic calix[4]reorcinarene with tris(hydroxymethyl) methylamide at concentrations of 10-6 (a, b), 10-7 (c, d), 10-8 (e) and 10-10 (f)
In both cases, subunits ~100 nm in diameter are evident in the supramolecular structures (see Figure 4 c, d). I have identified the subunits as coherent domains (CDs) formed in liquid water under ambient conditions from interaction with electromagnetic field as predicted by Emilio Del Giudice and his colleagues [2]. I have further proposed a general structure based on the near separation of charges in the CDs that turn them into effective dipoles, enabling them to aggregate into larger clusters when coherence in rotational levels become established among CDs through interaction with external electromagnetic fields. This is corroborated by Konovalov’s report that the presence of a 7 Hz electromagnetic field inside the permally container restores the formation of the supramolecular nanostructures.
The new results that nanostructures formation at high dilutions require electromagnetic fields and that these nanostructures are essential for biological effects are a major advance. They confirm and extend previous findings. The next step is for other laboratories to repeat the investigations, to further characterize the nanostructures associated with each solute, and to identify specific electromagnetic signals from the nanostructures that are also predicted from quantum electrodynamics field theory [2].
Article first published 06/10/14
Comments are now closed for this article
There are 14 comments on this article.
Berthajane Vandegrift Comment left 7th October 2014 00:12:52
I sounds fascinating, and I look forward to someone putting this into words that are understandable to laymen.
Robert M Davidson, MD PhD FAIS Comment left 7th October 2014 22:10:27
Very exciting research, Maewan! Thank you for sharing it. I'm curious as to the concentration of the nanoassociates formed.While the solutes may be present at extreme dilutions, the nanoassociates generated, are likely present at much higher concentrations, yes? Allopaths have trouble with the concepts of extreme dilutions and succusion, but these conditions may be necessary, in the presence of ELF EM fields, for the nanoassociates to form. Induction is likely facilitated by these conditions. Gilbert Ling's analogy to an "electromagnetic see-saw" may be helpful to the layperson.
algimantas k bronisas Comment left 7th October 2014 17:05:49
samuel hahnemans medical philosophy, in part , is certainly vindicated ....i publicly apologize to the homeopathic practitioners with whom i vehemently dis agreed.....especially mary castle....as i climb out of the allopathic black box
james Comment left 7th October 2014 17:05:37
Although I agree with Bertha Jane, I think I understand enough to have great hopes that, even though the conventional mechanistic approach to biological systems will take a long time to die, there is more reason for patients to believe that homeopathy works, and because of that belief there is a better chance for it work still. The mind can be a powerful contributor to healing.
mae-wan ho Comment left 7th October 2014 22:10:09
Hi Robert,
The concentrations of these aggregates would be about 50% of these dilute solution, according to quantum electrodynamics theory. I don't know what you mean by Gilbert Ling's electromagnetic see-saw; but I find Del Giudice's quantum electrodynamics theory most helpful. Please read previous articles cited.
Dana Ullman Comment left 8th October 2014 04:04:16
There is actually a much larger body of evidence for nanoparticles of the original medicine persisting in solutions than most people may realize. The article published in LANGMUIR (the publication of the American Chemistry Society!) has verified this with 6 substances that were tested with 3 types of spectroscopy, and the below 2 articles by Iris Bell, MD, PhD provide an excellent review of the growing body of evidence:
Chikramane PS, Kalita D, Suresh AK, Kane SG, Bellare JR. Why Extreme Dilutions Reach Non-zero Asymptotes: A Nanoparticulate Hypothesis Based on Froth Flotation.
Langmuir. 2012 Nov 1.
http://www.ncbi.nlm.nih.gov/pubmed/23083226
Bell IR, Koithan M. A model for homeopathic remedy effects: low dose nanoparticles, allostatic cross-adaptation, and time-dependent sensitization in a complex adaptive system. BMC Complement Altern Med. 2012 Oct 22;12(1):191.
http://www.biomedcentral.com/content/pdf/1472-6882-12-191.pdf (this is an exceptional review of the basic sciences literature that explains how homeopathic medicines may work)
Bell IR, Sarter B, Koithan M, et al. Integrative Nanomedicine: Treating Cancer with Nanoscale Natural Products. Global Advances in Health and Medicine, January 2014. 36-53.
http://tinyurl.com/mqe5p88
Robert M Davidson MD PhD FAIS Comment left 8th October 2014 17:05:36
I wonder why some nanoparticles are pathogens, e.g. Aluminum NPs, hemorrhagic fever viral NPs, whereas others seemingly demonstrate bio-benevolent properties, e.g. silver NPs, selenium NPs. Certainly, length scale (size) matters under the Lum-Chandler-Weeks theory. Regarding the effective concentration, if nanoassociates of water are roughly 50% by volume, in vivo,there would be a statistically high likelihood of such nanoassociates of biological water interacting, mediating, and perhaps amplifying the effect of the aforementioned pathogenic viral NPs and metal (Al, Hg, Pb) toxicant NPs. How might such an interaction occur?
Nhlonipho Gatyeni Comment left 8th October 2014 17:05:48
This is quite an amazing discovery . is it possible to have the same solutions being artificially formulated to incubate animal and plant organs?
maewan ho Comment left 8th October 2014 17:05:35
Hi Robert,
According to Del Giudice et al, ions are excluded from the coherent domains. However they respond to emfs through their cyclotron resonances. Please read the relevant chapter in my book Living Rainbow H2O where you can also find refs to the original papers.
Nhionipho, It is certainly possible to formulate such solutions to investigate their effects on cells and organs. It has been done. Please check Konovalov's papers for the references. It is important to use exactly the same procedure also to check that the nanostructures actually are there in the solutions.
Robert M Davidson MD PhD FAIS Comment left 9th October 2014 19:07:48
Do the nanoassociates described by Konovalov and by Shui-Yin Lo have identical properties to (a) Pollack's EZ water, (b) DelGiudice's CD water, (c) double helix water, and (d) electrolyzed-reduced water? Are these types of water synonymous? We might begin to be more precious in our definitions. Who was it first likened CD water to EZ water? It was Emilio, yes? Is there both information and energy content in biological water? Are there any methods by which we might externally "drive" or "pump" up the energy content of biological water in a bio-benevolent (safe) manner?
Robert M Davidson MD PhD FAIS Comment left 11th October 2014 11:11:26
What wavelengths does 7 Hz and its harmonics correspond to? Here's why I ask:
http://www.trueactivist.com/this-is-what-happens-when-you-run-water-through-a-24hz-sine-wave/
The 24 Hz sine wave, 23 Hz sine wave, and the 25 Hz sine wave in the water hose experiment apparently demonstrate HANDEDNESS, PERIOD, WAVELENGTH, PITCH, PHASE, and AMPLITUDE. They evidentally used sound waves to generate the macroscopic coherent helical water visual, yes? Could a similar phenomenon occur on the mesoscopic, microscopic, and nanoscopic length scales, under the scaling law? According to Wikipedia, "There is no rigid definition for mesoscopic physics, but the systems studied are normally in the range of 100 nm (the size of a typical virus) to 1000 nm (the size of a typical bacterium). 100 nanometers is the approximate upper limit for a nanoparticle." These dimensions are relevant for EMT, yes?
Robert M Davidson MD PhD FAIS Comment left 20th October 2014 16:04:21
Two "titans" of science have passed-away in 2014, Emilio DelGiudice and Masaru Emoto. A very sad year, indeed. Maybe it was their form of protest to what they see in the world, currently. The video clip and Emoto's book "Messages from Water" is very provocative. I don't claim to fully understand it. It made me wonder if the hexagonal motif has been recognized by x-ray crystallographers studying the solvation water of the DNA "double helix", perhaps as it supercools, prior to freezing. Maewan- Congratulations on your recent paper! Ho, M.-W. Illuminating Water and Life. Entropy 2014, 16, 4874-4891.Your Figure 2 captioned "Spherical coherent domains forming a three-dimensional dipole structure; note the six-fold symmetry resulting from the close-packing of spheres (from [23]" is extraordinary. The six-fold symmetry is that of hexagonal water, yes? IMO, the "real" genetic code lies in biological water. It possesses both information and energy. Helical water and double-helix water must have predated RNA, DNA, and life on Earth.
maewan ho Comment left 20th October 2014 16:04:03
Hi Robert,
according to QED theory of water, Coherent domains can respond to emfs to establish largescale coherence among domains and vortices may form. Thanks for your reference to my paper. Yes, I think snowflakes could form in a similar way from aggregates of CDs, especially as these CD-like aerosols have been detected near waterfalls.
Rosalind Walton Comment left 8th December 2014 19:07:35
I am a lay person who has been avidly following homeopathy for some years in part because I have chronic arthritis and get nasty reactions to allopathic drugs. I am delighted to see this type of research going forward. I hope that the knowledge that homeopathic have structure, coherence and produce electromagnetic signals can be widely disseminated. In the absence of this basic information the sceptics representing powerful vested interests have been free to bamboozle the public. They have been free to prevent homeopaths from curing ebola.