Mosquitoes engineered with a jumping gene vector to express a DNA-cutting enzyme produce >95 % male offspring; unfortunately both enzyme and vector target genomes of diverse species from slime moulds to humans Dr Mae Wan Ho
This commentary has been sent to the editors of the journal Nature Communications, inviting them and the researchers who have reported the creation of the new transgenic mosquitoes in the journal to reply
A team led by Andrea Crisanti at Imperial College London in the UK was widely reported to have made a breakthrough or even a ‘quantum leap’ in creating transgenic mosquitoes that could eradicate malaria [1]. Unfortunately, it is potentially the most hazardous genetically modified organism (GMO) to have been created, and should go no further from the laboratory. The researchers have not considered the risks involved, which would have been obvious from a casual review of existing literature.
Their fast-tracked online report in Nature Communications stated [2]: “Here we generate a synthetic sex distortion system by exploiting the specificity of the homing endonuclease I-PpoI, which is able to selectively cleave ribosomal gene sequences of the malaria vector Anopheles gambiae that are located exclusively on the mosquito’s X-chromosome. We combine structure-based protein engineering and molecular genetics to restrict the activity of the potentially toxic endonuclease to spermatogenesis. Shredding of the paternal X-chromosome prevents it from being transmitted to the next generation resulting in fully fertile mosquito strains that produce >95 % male offspring.”
Simply considered as a genetic trick, it is ingenious. Shredding the X chromosome of the male will make all of its offspring males. That is because female mosquitoes (like female humans) have two X chromosomes, one from the male parent and the other from the female parent, so without the contribution of the X chromosome from the male parent, only male offspring will result. A completely sterile male mosquito is useless, as it just dies out without affecting the population. But a fully fertile one that breeds exclusively males and pass on the sex-distorter trait would be ideal, as it would indeed wipe out the natural population, provided the trait is stably inherited. It would have been the perfect solution to destroying the natural populations of mosquitoes that transmit malaria; except that the DNA-cutting enzyme is by not means “specific” to “ribosomal gene sequences located exclusively on the mosquito’s X-chromosome” as stated. On the contrary, it cuts at a target sequence in ribosomal RNA (rRNA) genes - numerous copies of which are present in all eukaryote genomes – plus other sites as well, and the transgenic mosquitoes have been created using a jumping gene (transposon) vector that promiscuously invades all genomes. It is the female mosquitoes that bite people and transmit disease; so any transgenic female mosquitoes among the offspring would inject GM DNA containing the vector and I-PpoI transgene for horizontal transfer into people’s cells to shred their genomes.
The wild-type I-PpoI has been engineered into mosquitoes. The expression of the wild-type enzyme during spermatogenesis in transgenic mosquitoes causes cleavage of the paternal X chromosome, but also results in complete male sterility because the protein is stable and persists in mature sperm cells, leading to subsequent cleavage of the maternal X chromosome in the zygote after fertilization, thereby killing the zygote. Thus, the sterile males would just die out without leaving any offspring, and not make any difference to the natural population, unless they are continually released into the wild.
To overcome that, the team mutated amino acid residues in the hydrophobic core of the endonuclease to obtain recombinant proteins with reduced stability. The melting temperature of the protein was decreased from 54.4 °C in the wild type to 49.4 °C in mutant L111A and 35.1 °c in a double mutant L111A/W124L. The thermal half-life at 37 °C ranged from 73.5h for the wild type enzyme to 2 h for H106A. The L111A/W 124L double mutant had a half-life of about 4 min. The specific activity of the wild type was ~7 pmol/min/mg compared to about a third of that in mutant H106A.
Germ line transformation constructs were created to express the I-PpoI variants placed under the control of the male spermatogenesis-specific b2-tubulin promoter. The constructs were designed to express both enhanced green fluorescent protein (EGFP) as in frame fusion protein or, using a 2A ribosomal stuttering signal, as distinct protein chains. The constructs were put into the promiscuous piggyBac vectors for transgenesis. Constructs that had integrated on the X chromosome failed to show significant levels of I-PpoI expression in the testes, probably because the X chromosome is transcriptionally silent during male meiosis.
Reduced in vitro thermal stability translated into significantly lower protein levels of EGFPI-PpoI protein in vivo. The destabilized I-PpoI distorts the sex ratio towards males as measured in emerging adults, fertility was measured by larval hatching rate and number of eggs in crosses of transgenic male mosquitoes to wild type females. As a control transgenic female mosquitoes of each strain were crossed to wild type males. Male mosquitoes expressing the wild-type enzyme were sterile as previously found. No effect on fertility or sex ratio was observed in all strains expressing the double mutant L111A/W124L, which had the lowest in vitro stability; or in strains carrying X-linked transgenes. Significantly, male biased sex ratios ranging from 70.2 to 97.4 % were found in the progeny of males carrying the remaining I-PpoI variants; of those, only three lines, 111A-2 and 124L-2, 124L-3 had comparable hatching rates to the wild type. These strains also showed the lowest mRNA levels. The sex distortion phenotype was stably inherited from male mosquitoes to their transgenic sons. For four subsequent generations, 111A-2 fathers showed comparable levels of fertility and male-biased sex ratios in their offspring. Homozygous males in strains 111A-2 and 124L-3 caused a reduction in the hatching rate, but had no effect on fertility in 124L-2.
It is important to note that the sex distortion is not complete, which means that a variable number of transgenic females will be left to bite people and transmit the potentially lethal transgenes into people’s cells. Such transgenic females were found to have more female offspring when mated to wild type males (see below). In the wild, the proportion of surviving transgenic females, and indeed, I-PpoI-resistant transgenic females could also arise (see below). All that would make it more likely to spread the transgenes into human and other mammals.
Female offspring of transgenic male mosquitoes showed evidence of misrepair and copy number variation in the ribosomal gene cluster in their genome, suggesting chromosomal damage. There was also a significant female-biased sex ratio in the progeny of female survivors crossed to wild type males suggesting that loss of vitality occurred in individuals that had inherited only a damaged X chromosome from the transgenic female.
But have the transgenic female offspring inherited integrated I-PpoI endonuclease genes in the X-chromosome, which would make the chromosome resistant to further endonuclease damage (as integration destroys the target site, see below)? To test for this possibility, the transgenic females were crossed to wild type males, and the transgenic male progeny, which have a 50 % chance of carrying the potentially resistant X-chromosome was then crossed to wild type females, and the sex ratio of individual crosses was recorded. The analysis showed a male bias in the progeny of all males that did not differ significantly from the 111A-2 stock, suggesting that the X chromosomes of female survivors are damaged but still susceptible to further cleavage.
In five independent cage experiments, the release of hemizygous 111A-2males at a ratio of 3X controls was effective in supressing caged wild type populations (achieving elimination within 6 generations in four out of five cages. As expected the release of sterile males expressing the wild-type enzyme had no measurable effect in three populations. Sex distorter is much more effective than sterile males by 2 orders of magnitude, and 2-70 times more effective than equivalent releases of female-killing alleles.
This sex-distorter system is clearly an improvement on the Oxytec transgenic mosquitoes engineered with an ill-characterized, ineffective, as well as hazardous system [3, 4] (Regulation of Transgenic Insects Highly Inadequate and Unsafe, Transgenic Mosquitoes Not a Solution, SiS 54). There is an alternative non-transgenic system based on a common symbiotic bacterium that can stop the dengue virus multiplying in the mosquito host, which effectively makes those transgenic mosquitoes obsolete [5] (Non-transgenic Mosquitoes to Combat Dengue, SiS 54). But the ill-advised Brazilian government has approved Oxytec’s transgenic mosquitoes for commercial release in April 2014 [6].
In terms of safety, the new sex distortion transgenic mosquito is no better and possibly worse.
The homing endonucleases are a collection of enzymes encoded either as freestanding genes located within introns (intervening noncoding sequence in protein-coding genes), as fusions with host proteins, or as self-splicing inteins (sequences within proteins that splice themselves out after translation). They cut genomic DNA within the cells that synthesize them at target sites. Repair of the cut DNA by the host cell frequently results in the gene encoding the homing endonuclease being copied into the cleavage site, hence the term 'homing' to describe the movement of these genes. Homing endonucleases can thereby transmit their genes horizontally within a host population, increasing their frequency more rapidly than classical Mendelian genes [7, 8].
Homing endonuclease (HEN) recognize target sequences of 15 to 40 bp long. Generally, owing to the homing mechanism, the gene encoding the endonuclease is located within the recognition sequence which the enzyme cuts, thus interrupting the homing endonuclease recognition sequence and limiting DNA cutting only to sites that do not (yet) carry the HEN. However, target recognition is not completely specific, and off target cleavage and integration has been known at least since the 1990s.
The I-Ppol homing endonuclease used in the construction of the sex distorter transgenic mosquitoes [1, 2] was originally isolated from the slime mould Physarum polycephalum. It is a member of the His-Cys box family found in myxomycetes and amoebae. The His-Cys box protein motif consists of two conserved histidine and three conserved cysteine residues within a 30 residue region of protein. These conserved His and Cys residues appear to contribute to a Zn2+-binding motif and to the endonuclease active site. The I-Ppol target site of 15 bp and cleavage position is as follows [9]. The cleavage at TTAA leaves a 4 nucleotide overhang at the cut ends.
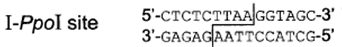
However, a substantial degree of degeneracy of the target sequence appeared to be tolerated, as demonstrated in a study in which partially randomized cleavage sites were created by mutagenesis [9]. Not only that, multiple target sites exist in all eukaryote genomes.
The native I-PpoI target site is by no means exclusive to the mosquito X-chromosome, it is actually present in multiples copies in the highly conserved 28S ribosome RNA (rRNA) genes of all eukaryotes species including humans, and currently being deployed in gene therapy experiments [10]. Each diploid human cell has about 600 copies of the rRNA genes in five clusters located to the short arms of chromosomes 13, 14, 15, 21, and 22. On account of the multitude of rRNA genes and the presence of possibly inert spacers between the gene repeats, the rDNA is considered an appealing safe haven for transgene integration. A team of researchers in Finland have created an HIV-1 integrase-1 PpoI fusion protein in order to guide the integration of an HIV viral vector into the rDNA [10].
The team also carried out a study on cytotoxicity of the construct. Although a genome-wide interaction study found that the IN-fusion PpoI proteins bind to their target sequence containing 28 S rRNA genes with 100-fold enrichment compared to controls, there was significant off-target binding to non-rRNA gene sites, and considerable cytotoxicity for all human cell lines tested from the double-stranded breaks in DNA produced by the I-PpoI endonuclease [11]. The researchers put a positive gloss on the findings by noting that the construct was more toxic to cancer than normal cells, and suggesting that the endonuclease could be used for cancer therapy.
I have long warned against using promiscuous transposons as gene transfer vectors, especially for insects that bite people [12] (Terminator insects give wings to genome invaders, I-SIS report). The piggyBac transposon was discovered in cell cultures of the moth Trichopulsia, the cabbage looper, where it caused high rates of mutations in the baculovirus infecting the cells by jumping into its genes [13] (Terminator insects – a primer, ISIS Report). The piggyBac is 2.5kb long with 13 bp inverted terminal repeats. It has specificity for sites with the base sequence TTAA (same as the I-PpoI endonuclease cleavage site, see above). The probability of this sequence occurring at random in any genome is 0.254 or 0.4%. There is also evidence that the disabled piggyBac vector carrying the transgene, even when stripped down to the bare minimum of the border repeats, was nevertheless able to replicate and spread, because the transposase enzyme enabling the piggyBac inserts to move can be provided by transposons present in all genomes. The main reason initially for using transposons as vectors in insect control was precisely because they can spread the transgenes rapidly by ‘non-Mendelian' mean within a population, i.e., by replicating copies and jumping into genomes, thereby ‘driving’ the trait through the insect population. However, the scientists involved neglected the fact that the transposons could also jump into the genomes of the mammalian hosts including human beings. Although each transposon has its own specific transposase enzyme that recognizes its terminal repeats, the same enzyme can also interact with the terminal repeats of other transposons, and evidence suggests extensive cross-talk among related but distinct transposon families within a single eukaryotic genome (reviewed in [3]).
The use of the piggyBac transposon has been plagued by problems of instability in transformed Aedes aegypti [14]; and large unstable tandem inserts of the piggyBac transposon were prevalent [ 15]. In spite of instability and resulting genotoxicity, the piggyBac transposon has been used extensively also in human gene therapy [16]. Several human cell lines have been transformed, even primary human T cells using piggyBac [17]. These findings leave us little doubt that the transposon-borne transgenes in the transgenic mosquito can transfer horizontally to human cells. The piggyBac transposon was found to induce genome wide insertion mutations disrupting many gene functions.
Transgenic mosquitoes are not the solution to eradicating dengue or malaria. On the contrary, they are among the most hazardous GMOs created, and should never be released into the wild on any commercial basis.
Researchers should consider the risks involved before embarking on a project, and science journal editors and commentators should also question whether works for publications carry risks to health and the environment.
Article first published 24/06/14
Comments are now closed for this article
There are 18 comments on this article.
Trav Comment left 26th June 2014 14:02:43
These GMO mosquitoes are already released in Australia and various other countries around the world and are likely to have been caught in shipping containers which would have reached other countries.
Bill Gates is partially responsible for this as he helped fund this in Australia, he is PURE SCUM.
Paulo Andrade Comment left 29th June 2014 14:02:55
DDear Sirs.
You wrote "... a variable number of transgenic females will be left to bite people and transmit the potentially lethal transgenes into people’s cells" How, for God´s sake, mosquito genes could be transferred to human beings? The genes and the new proteins are within the insect cells, which are not injected in the animal serving as food supply. Could you please clearly explain the mechanism of such transference, its likelihood and the associated damage and risk? As it is written, it is pure and useless speculation.
mae-Wan Comment left 29th June 2014 21:09:07
Paulo,
When a mosquito bites, it constantly injects saliva into your blood to keep the blood from clotting, that's how it transmits the malaria parasite, as well as any of its cells dead or alive and nucleic acids, which will contain the GM constructs that could be taken up into your cells. This is horizontal gene transfer, the very process exploited in creating transgenic cells and organisms.
You are right it is still hypothetical in the case of transgenic mosquitoes. The researchers should find out if the experimental animals (mice?) used in feeding blood to transgenic mosquitoes get transgenes transferred into them as part of risk research. But this has never been done.
Brad Forsythe Comment left 30th June 2014 16:04:36
Paulo, How do you know the time span is not comparable to an evolution - you cannot guarantee the lifespan of genes in breeding populations? As far as i understand even terminator genes (which i assume aren't in these mosquito populations) would still be subject to an evolutionary change. An how long is an evolutionary time span when we are talking about insects in their indefinably large population numbers and breeding cycles that allow several generations per year?
Paulo Andrade Comment left 30th June 2014 18:06:53
Dear colleague, I did you not watch for your reply, as I was celebrating the victory of the Brazilian National Soccer Team.
As a parasitologist, working all my life with leishmaniasis and Chagas´disease in Brazil andelsewhere, I am fully aware of the mechanism by which mosquitoes and other arthropods suck blood and eventually transmit a virus, a parasite or a bacterium. In spite of a million years of injecting their (degraded) DNA and those of their infectious agents, we can´t find mosquito, Plasmodium, Leishmania or any other exotic genes or gen fragments in our genome. This is the main proof that horizontal gene transfer is a very rare event among any species and a higher eukaryote.
The mechanisms of transfection, being highly artificial, bear no resemblance to natural horizontal flow of genes and it is illegitimate to compare both things. There is a huge bulk of evidences pointing toward the implausibility of horizontal gene transfer between any species and an eukaryote and there is no reason to experiment on that or to speculate that such a mechanism could pose a non-negligible risk to humans from transgenic blood sucking arthropods(remember, risk assessment must indicate that a pathway to harm exists and that a non-negligible likelihood can be determined, what is surely not the case).
Moreover, piggyback transposons are not worst than virus because they do not carry diseases and are unable to transmit themselves between cells, as virus do. Moreover, to be transposed, they need the transposase they code. The transgenic constructions do not carry the gene for transposase and they will not get the correct transposase from their new host (Anopheles, in this case). That means the construction will stay in the same position where it was originally inserted.
Finally, in the former blog where I posted my questions and got the answers, there was another question regarding the lifespan of genes in the breeding population and the time needed for evolution to allow gene exchange by (the very unlike) horizontal transfer. This was already commented before, but I will ass some additional info: it is irrelevant how long the transgenes will survive (the rest of the Earth lifespan, for example), because in the last million years during which mosquitoes and mammalian hosts lived together, this speculative horizontal transfer never happened, even in the case of transposable elements from arthropods and from their infective agents. Again, the risks are negligible.
MaeWan Ho Comment left 30th June 2014 18:06:02
Paulo,
When I first raised the dangers of horizontal gene transfer 10 years ago, I was indeed criticized by scientists who said horizontal gene transfer (hgt) is an extremely rare event. However, very soon, it became clear that hgt has been rampant during evolution, and can be seen to happen both in the lab and in the field. So they say it is a natural process, and no need to worry. Unfortunately, GM constructs are designed precisely to make it happen more easily, they are designed to overcome barriers and to invade genomes. I have written an entire book on the new genetics, and a series of new articles on the subject. Please start with Horizontal of GM DNA Widespread but No One is Looking, almost here: http://www.i-sis.org.uk/Horizontal_Transfer_of_GM_DNA_Widespread.php, and a paper, The New Genetics and Natural versus Artificial Genetic Modification, which is published and available on open access here: http://www.mdpi.com/1099-4300/15/11/4748
Paulo Andrade Comment left 2nd July 2014 03:03:58
Dr. Ho, please allow me to disagree with some of your statements. HGT has not “been rampant during evolution”. It happens in very low frequency, as can be seen by inspecting the ever growing number of fully sequenced genomes for the three kingdoms; but, by no means is common, especially between eukaryotes. It is not correct to generalize the findings on HGT from bacteria and other simple, single cell organisms, to metazoan and there is no evidence that such HGT never happened, even within the broad lifespan of Evolution. Therefore, the first step in the pathway to harm has a very, very low likelihood to happen and, therefore, we can´t reasonably admit that the risks will be non-negligible. Moreover, even if the construction of this GM mosquito were transferred to a human being, it would have no influence on his (or her) metabolism and would not be transmitted to their offspring. The simple presence of a new gene (or transgene) is, per se, not a harm: it must trigger metabolic changes, what is highly improbable for the mosquito genes and also for the transgene (different promoters, different targets, etc.). Therefore, the second step of the pathway to harm is also very unlike. Finally, the third step (the harm being distributed in a large non-target population) is also very unlike, as only a small percentage of females are released and only a fraction of them will suck human blood. Moreover, the new gene should be incorporated in a rapidly growing cell, which is very unlike in the peripheral blood and being transmitted to the person´s offspring. The next events are also improbable until the final harm. Therefore, in my point of view (based on hundreds of risk assessment I did myself or read in the last seven years), there is an absolutely negligible risk that such technology may harm human beings or other vertebrates.
In conclusion, when you say that the new technology “is a good trick, but no consideration of risks” you are supposing that the scientists who develop the technique did not question themselves on safety aspects related to the release of these GM insects. They did and came to the same conclusions I reached above, based on what we have in the real world of genetics, The future may prove that you are right and I (and the other scientists and gov´ risk assessors) are wrong, but meanwhile we should not ban biotechnology.
MaeWan Ho Comment left 2nd July 2014 03:03:46
Paulo, thank you for your fulsome reply. You may be right that horizontal gene transfer between eukaryotes are rare compared to that between prokaryotes, especially for multicellular eukaryotes. However, horizontal transfer between plants, for example, could be mediated by insects that bite plants. Afterall, viruses are transferred between plants by aphids and other insect vectors, in the same way that insects that bite people can transfer viruses and parasites between people. Obviously, the horizontal transfer of genes carried by insects would be much more difficult to detect, simply because there are too many other cells present. You may also be right that these events are rare in evolution, though we still do not have enough multicellular genome sequenced to conclude that is the case. Signs are that metazoan genomes are much more permissive to horizontal gene transfer, that is why they have taken up numerous genes from prokaryotes and archaea bacteria in the course of evolution, and they do not require homologous recombination. Moreover, transgenesis is precisely designed to overcome natural barriers to horizontal gene transfer, hence, as you admit, we cannot rule out the possibility for horizontal transfer of transgenes to happen; piggBac is a very efficient gene transfer vector now used in gene therapy. Further, your assumption that the transgene itself is harmless is contradicted by the cytotoxic effects found experimentally in all the human cells tested. I am therefore not convinced by your risk assessment.
Other people have raised the issue of the ecological impact of making natural populations of mosquitoes extinct, and whether other methods of disease control such as old fashioned mosquito nets and improved sanitation would be preferable to creating transgenic mosquitoes in the first place.
Paulo Andrade Comment left 2nd July 2014 20:08:52
Dr. Ho, here are again some considerations on HGT. You said that “horizontal transfer between plants, for example, could be mediated by insects that bite plants‘. Even if some cells or DNA could be transferred from a plant to another, there is
a) No reason to believe that the cells would find a way to transfer some genes to the new “host”. Even if this happens, the cell should generate a seed, what is highly unlike or even impossible.
b) No reason to believe that the DNA would cross the thick plant cell walls and find its way in the cell nucleus. Even if does such an unlike movement, it should then be inserted in the new genome, again a very rare event if not purposely done using DNA flanked by integrating sequences specially designed for the host genome.
The fact is that insects are biting and feeding on plants for 400 million years and there is absolutely no evidence of such transfers (by HGT) among plants. I must conclude that the idea of such transfers is attractive, but not supported either by theory or by observation.
You also said: “Signs are that metazoan genomes are much more permissive to horizontal gene transfer, that is why they have taken up numerous genes from prokaryotes and archaea bacteria in the course of evolution”. Firstly I should correct that cells are permissive to HGT, not their genomes. Second, the eventual presence of very few bacterial, viral or other non-eukaryote genes in higher eukaryotes genomes is by no means a proof or even an evidence of horizontal gene transfer. There are many other mechanisms that have been proposed which are more likely. Moreover, as you now, eukaryotes probably derived from a very early fusion between eubacteria and archea and this is one of there reasons we have sometimes old traces of this event in our genomes.
You also said: “Transgenesis is precisely designed to overcome natural barriers to horizontal gene transfer” I must disagree: transgenesis is a way to overcome the limitations of VERTICAL gene flow in conventional crop breeding. Molecular biologists are still working hard to transfect plant or mammalian cells in an efficient way, again proving that HGT is very difficult even in well controlled lab conditions. Therefore, contrary to your conclusion, I must say as most scientists do: HGT is a very rare event in Nature, especially among eukaryotes.
Moreover, you said that transgenes are deleterious when transferred to human cells. I agree that the expression (not the simple presence) of a transgene can be deleterious for a cell, but it will depend on the gene and on the cell. There is absolutely no reason to believe that the expression of the proteins encoded in the mosquito construction will be deleterious for a human being. The idea that any transgene, either expressed or not in the new host cell, would be deleterious, is somewhat naïve, as can be easily observed by the success of transgenic mice, rats, plants, microorganisms, fish and viruses.
As for the efficiency of piggyback transposons vectors, it is still under study and far from being used for gene therapy. Anyway, there are hundreds of such vectors and those used in mosquitoes have no reason to perform well in human or plant cells.
Being one of the risk assessors for the GM mosquito in Brazil, I faced the questions you and many others raised in the last three years, including the highly speculative and non-operational idea that other worst arthropod vectors would feel the vacant ecotones after a successful vector control campaign (this is not a biosafety concern, however). For every hazard we constructed a pathway to harm with all the necessary steps to connect the hazard to the final harm. My colleagues and me found no specific risks for the GM mosquitoes. Anyway, as indicated by our regulatory system, we are going to monitor the commercial release of these mosquitoes in the next year. Other transgenic insects will possibly be evaluated by the Brazilian CTNBio, always in a case by case approach and using the robust risk assessment guideline adopted by most risk assessment agencies in the World. Your concerns and others will always be taken into account and submitted to the scientific scrutiny I explained above. Eventually, most of these concerns will be discarded and the remaining ones (those having a real pathway to harm) will be used to classify risks and to help our government take a science-based decision.
Finally, risk assessment has the sole mission to point to risks. The cost/benefit ration and other economic, social or cultural questions belong to risk analysis. In my country this is the duty of the National Biosafety Council, composed of 11 State Ministers. If CTNBio says risks are negligible or can be controlled and mitigated, the NBS may decide that the product can´t be used for economic, social, technical or cultural reasons, but no because of biosafety considerations.
Kindly
Paulo Andrade
Maewan Ho Comment left 2nd July 2014 20:08:56
Paulo, on HGT, are we reading the same literature? Here is a review published in 2005, Lateral gene transfer in eukaryotes: http://www.ncbi.nlm.nih.gov/pubmed/15761667 , in the abstract, its says: “Lateral gene transfer… has been acknowledged as a major mechanism in prokaryote genome evolution for some time. Recently accumulating data indicate that the process also occurs in the evolution of eukaryotic genomes….Transfers between eukaryotes also occur, mainly into larger phagotrophic eukaryotes that ingest eukaryotic cels, but also between plant lineages….” Insects can definitely penetrate plant cell walls, because they have sharp mouth parts.
It is well-known that the eukaryote genome has many prokaryotic genes as well as Archaean genes, and a 2014 article, Horizontal gene transfer in the acquisition of novel traits by metazoans “reviews the rising evidence on horizontally transferred genes and on the acquisition of novel traits in metazoans…”: http://rspb.royalsocietypublishing.org/content/281/1777/20132450.full.pdf+html.
Both refer to evolution, and hence under represent horizontal gene transfer in real time, which need not register in evolution, particularly if a cytotoxic effect kills the cells or organisms into which the transfer is made.
On biosafety, I was actually among the scientists involved in the early negotiations on the Catagena Protocol on Biosafety, as well as a member of their roster of experts. Biosafety considerations do most certainly include environmental and socioeconomic impacts as we;; as health impacts. It also includes the precautionary approach. It would be unfortunate if these have been left out of consideration by the risk assessments in Brazil.
And now, I must bring this discussion to a close because I have many other pressing things to attend to. But we have aired the issue sufficiently, and most importantly, in terms that the public and our policymakers, as well as other scientists can understand. I would like to thank you again for commenting in such a civil and rational manner; I am not unaware that commenting on my article puts you at a disadvantage, which I am sure others will recognize and appreciate as much as I do.
Raul Picaporte Comment left 7th September 2014 05:05:15
There has been a recent outbreak of the Chikungunya virus in Puerto Rico. The virus practically breaks you. Symptoms of pain may last years. It is said the virus was made in a lab and released into the wild as a biological weapon. What is suspicious to us here is we'd never heard of the disease until now. If there is a known cure it isn't available. The assertion that because it is lab-made there is no "natural" cure is questionable. This article suggests that, apart from it's immediate consequences on human and animal health, the possible transgenic nature of the virus poses unprecented challenges for environment. However brilliant certain inventions may be from a technical aspect, we are limited in intuiting the butterfly effect in detail. Our degree of responsibilty, coupled with our inherent perceptual limitations, should advise us to use our power with care and restrictions.
Brian Sandle Comment left 10th November 2014 18:06:47
I would appreciate comment on this video about apparently very effective single dose cure of malaria with a rather weak solution of chlorite and activator. https://www.youtube.com/watch?feature=player_embedded&v=5jY2yab0uLc
Jane Comment left 2nd February 2016 02:02:16
Sadly, Dr.Ho, your conclusions have been proven correct. To the horror of the people of Brazil.
? Comment left 13th February 2016 08:08:43
Dr Mae Wan Ho, thank-you for the informative debate on (HGT), I look forward to reviewing the articles cited in your comments.
Something that may interest you or or maybe not ? Genetics & Microcephaly as it refers to the Zika virus & genetic manipulation.
http://www.avianflutalk.com/genetics-microcephaly_topic35398.html?SID=1145219cceca8eae27e766bae9afd4459027778
? Comment left 13th February 2016 08:08:22
Dr Mae Wan Ho thanks for the information on possible problems with HGT via Oxitec's GM Mosquitoes, I look forward to reading your articles.
You might find the following of interest maybe, maybe not? >>> Genetics & Microcephaly http://www.avianflutalk.com/genetics-microcephaly_topic35398.html?SID=1145219cceca8eae27e766bae9afd4459027778
Susan Rigali Comment left 17th May 2016 21:09:58
Thank you, Dr. Mae Wan Ho you have taught so many in this world about life. I was crushed to hear of your illness. You have been a patient scholar, a force for nature, and your intelligence with care surpass most all in the scientific community. Thank you for defending other colleagues that were ostracized all while continuing your work both scientifically and creatively. I come here often and pass your words with recognition for your accomplishments. Amazing woman! I also thank Juan Surkula for directing me to your site 20 years ago.
Susan Rigali Comment left 22nd September 2016 23:11:18
Thank you, Dr. Mae Wan Ho you have taught so many in this world about life. I was crushed to hear of your illness. You have been a patient scholar, a force for nature, and your intelligence with care surpass most all in the scientific community. Thank you for defending other colleagues that were ostracized all while continuing your work both scientifically and creatively. I come here often and pass your words with recognition for your accomplishments. Amazing woman! I also thank Juan Surkula for directing me to your site 20 years ago.
Colleen Ryer Comment left 22nd September 2016 23:11:10
Excellent article.
Oxitec entered into a mosquito testing agreement with Malaysia in 2006.