Graphene and carbon nanotubes shaped into supercapacitor fibres by wet-spinning liquid crystal mesophases and other means for whole new generations of microelectronics and macroscale wearable applications Dr. Mae-Wan Ho
Fibre supercapacitors are shaped as one-dimensional wires with diameters ranging from micrometres to millimetres, generally small, lightweight and very flexible. They can be woven or knitted into fabric/textiles for wearing, and their unique architectures also make for great versatility in design, as they can be made practically into any desired shape and located anywhere [1]. Fibre supercapacitors have potential applications in two areas: as micro-scale devices to complement or even replace micro-batteries in miniaturized electronics or microelectric mechanical systems; and as macro-scale devices for flexible wearable electronics or smart fabrics/textiles. They could be directly integrated/interwoven with various energy harvesting devices – for heat, light and mechanical energy - that are also developing rapidly in micro-planar and micro-fibre configurations.
In fibre supercapacitors too, carbon materials have proven superior as electrode material, with its high specific surface area, better electrical conductivity, high rate capability, better cyclic stability, and not least its ability to play host to a wide range of guest molecules to extend operating voltage and energy density.
Graphene is a 2-dimensional material with exceptional mechanical, electronic and chemical properties and hence a wide range of potential applications (see [2, 3] Why Graphene is Amazing, Graphene & the New Age of Carbon and other articles in the series, SiS 59). The challenge is to develop methods to take advantage of these remarkable properties that can assemble the single-atom thick carbon sheets into macroscopically structures, in particular, macroscopically ordered structures.
Material scientists Zhen Xu and Chao Gao at Zhejiang University, Hangzhou, China, have risen to the challenge and managed to create a range of multifunctional 1-dimensional graphene fibres by wet-spinning graphene liquid crystal colloids either neat or mixed with additives that can be incorporated into the same fibre or in layers around it [4].
First they created highly dispersible derivatives of graphene so they can be dispersed in favourable and environmentally friendly solvents. Then they identified soluble lyotropic liquid crystalline graphene derivatives, i.e., liquid crystals that undergo phase transitions as concentration is increased. Finally, they used the industrial process of wet spinning – usually applied to polymers – to shape the liquid crystals into a range of graphene fibres with or without hybridization with other materials.
The usual method of preparing graphene (G) starts with the exfoliation of graphite after oxidation into graphene oxide (GO). This is important as GO is much more dispersible than G. And also, by selecting the size of the graphite block for exfoliation, the size of the resulting sheets can be controlled, from submicrometres to several micrometres up to dozens of micrometres or 100 mm.
Graphene itself is not readily dispersible except in harsh chlorosulfonic acid. Thus it is only in GO that useful mesophases exist. Xu and Gao discovered a rich variety of nematic (thread-like) and lamellar (sheet-like) mesophases in GO in 2010. GO dispersions are typically lyotropic in that increasing concentration lead to the formation of mesophases. Thus, GO (D~2.1 mm) dispersions began to exhibit liquid crystalline birefringence upon increasing to 3 mg/cm3 and formed stable mesophases at 6-8 mg/cm3 under the polarized light microscope. The lammellar mesophase of GO was detected on increasing the concentration above 10 mg/cm3. Since 2011, the GO nematic LC family has been extended to include GO sheets with lateral width from submicrometres to several micrometres and giant GO dozens of micrometres, as well as GO ribbons. The larger the size, the higher the aspect ratio, the lower the critical concentrations at which phase transition begins; for example, the critical concentration is 0.04 vol % for GO with aspect ratio above 104. The more narrow the size distribution, the narrower the concentration range for phase transition, and the more regular the alignment.
A new chiral liquid crystal was discovered in GO sheets system as concentration further increased to 0.38 vol % following the nematic phase at 0.23 vol %. It has a helical lamellar structure with strong optical activity.
To create further liquid crystalline mesophases, functional groups can be added to GO. There are two categories of chemically active sites on GO: the oxygen containing groups and the C=C double bonds. The first includes hydroxyl, epoxy, and carboxyl groups that allow small and giant molecules to be grafted onto GO basal planes and defective edges to weight fractions of 19-50 %. These oxygen containing sites could also serve as nucleating points for multifunctional graphene hybridized by nanoparticles with tunable density and size. GO and chemically reduced graphene (CRG) were physically functionalized with both linear and dendritic polymers such as PVA (poly (vinyl alcohol)) and hyper-branched polyglycerol (HPG) via hydrogen-bonding. The adsorbed polymer content up to 64 wt % enabled CRG to be well dispersed as single layers in water and polar organic solvents at high concentrations (>50 mg/cm3).
The C=C double bonds reactive sites involve free radical chemistry, such as nitrene chemistry and free radical polymerization. In the case of nitrene chemistry, small molecules and polymer chains with active azido (N3-) groups were covalently grafted onto GO to form cyclic compounds with the C=C bonds at high temperature, which resulted in in situ thermal reduction of GO simultaneously. As to the free radical polymerization, a family of fibrous graphenes (FGs) grafted by more than ten kinds of vinyl polymers was established, from hydrophilic PSSNa (sodium sulfonated polystyrene) to hydrophobic PS (polystyrene), and from amorphous PMMA (poly(methyl methacrylate)) to crystalline PAN (polyacrylonitrile). The high density grafting of polymeric chains (1.6 × 104 chains/μm2) rendered graphene finely dispersible in water and organic solvents, keeping stable in single layer state up to high concentrations (>15 mg/cm3). The polymer-grafted graphenes are expected to be well-compatible nanofillers for universal polymers, and also interesting new building blocks of 2-D brushes for constructing graphene macroscopic materials.
Besides promoting liquid crystal formation, graphene chemistry allows a tuning of the chemical composition for design of interlayer interactions.
“Graphene fiber is the much dreamed [of] macroscopic material of graphene in the 1D form.” Xu and Gao stated. By fluid assembly, the continuous alignment of graphene in the solid state can be deduced from the ordering in the fluid state. Wet-spinning resulted in a rich family of graphene-base fibres, including neat GFs, biomimetic composite fibres and hybridized fibres (Figure 1).
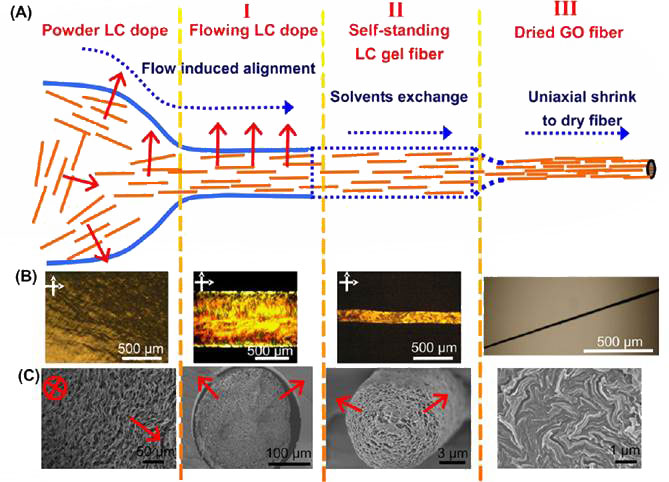
Figure 1 Wet-spinning liquid crystalline graphite oxide into fibres; A. enhanced alignment under uniaxial flow; B, C, tracking the stages with polarized and scanning electronmicroscopy respectively on the fibres
The procedure is based on the same principle of wet-spinning used for polymeric fibres. Liquid crystalline GO dispersions are continuously injected into coagulating baths to generate continuous GO fibres. After chemical reduction, they turn into graphene fibres with the same orientation ordering of the flowing GO Liquid crystalline dopes. These fibres are mechanically strong and flexible so that they can be fastened into tight knots without breaking, and integrated into conductive patterned textile with other threads. Cross-linking with Ca2+ enhanced mechanical strengths up to the record 412 MPa at 3.2 % elongation and 501 Mpa at 6.7% elongation respectively. At the same time, electrical conductivity increased to 3.8-4.1 x 104 S/m. Thermal annealing also improves mechanical strength.
By controlling the coagulation conditions and nozzle types, special porous GFs could be made. Graphene aerogel fibres were made by using liquid nitrogen as coagulation bath followed by freeze-drying. These have aligned pores in the core and a densely compact shell. The unique core-shell structure and regular alignment of GO sheets resulted in high specific tensile strength (188 kN m/kg), fine compress strength (3.3 Mpa) and high specific surface area (884 m2/g). In addition, the pores inside offer space for functional guest particles or polymers.
Controlling the flow, coagulation and final drying of the fibres is very important for getting good quality fibres. The highest Young’s modulus (up to a record 47 GPa) and tensile strength (up to 0.5 GPa) has been obtained with GFs with stretching during wet-spinning.
Coaxial spinning of GO dispersions has created continuous coaxial GFs with graphene core and polymer shells or CNT core and graphene shell for use as yarn supercapacitors. Particularly important are families of ordered composite and hybridized graphene fibres.
Chao Gao and a team of researchers at Zhejiang University have indeed created fibre supercapacitors using coaxial wet-spinning assembly [5]. Three kinds of coaxial fibres were spun and used as supercapacitors. The spinneret is equipped with two inlets connecting to the inner and outer channels respectively.
The first coaxial fibre was graphene oxide (GO) coated with carboxymethylcellulose (CMC), GO@CMC. The GO@CMC fibres were chemically reduced by hyroiodic acid, changing the colour of the core from brown to black. The chemically reduced RGO@CMC fibres were highly conductive with conductivity ~ 7 000 S/m as measured from their naked ends, comparable to neat graphene fibres and papers (~ 7 000-25 000 S/m), confirming the integrity of the inner fibre, enabling it to be used as an electrode for fibre supercapacitor. Ultralong coaxial fibres up to 100 m were achieved. Under SEM, the GO@CMC shows a fibre with a GO core of ~ 50 mm and a CMC sheath ~ 25 mm.
The second was spun with carbon nanotube (CNT) as the core resulting in CNT@CMC fibres. The thickness of the sheath is ~2-5 mm and the diameter of the CNT core ~ 50 mm.
The third used a mixture of RGO and CNT as core, resulting in RGO+CNT@CMC fibres. The CNTs were coated on the surfaces of RGO densely and separated the adjacent RGO sheets aligned with layered structures. The coating greatly increases the surface area of the fibres, which is beneficial for energy storage.
The coaxial fibres were tightly intertwined in pairs then coated with a layer of H3PO4PVA gel electrolyte as the ion source. The RGO@CMC, CNT@CMC, and RGO+CNT@CMC supercapacitors were tested with the standard techniques (see [6] All Carbon Graphene Supercapacitors Coming Part 1, SiS 66). Cyclic voltammetry was performed at scan rates of 10 to 200 mV/s, and charge discharge curves obtained at current densities of 0.1 to 0.8 mA/cm2. The volume capacitance for RGO@CMC, CNT@CMC, and RGO+CNT@CMC were 114 F/cm3, 42 F/cm3 and 158 F/cm3 respectively at a current density of 0.1mA/cm2; that of RGO+CNT@CMC approximately the sum of the previous two. This suggests that the RGO+CNT@CMC supercapacitor corresponds to the parallel connection of one RGO@CMC supercapacitor and one CNT@CMC supercapacitor. Also, RGO+CNT@CMC appears to have the high rate capacitance of CNT@CMC retaining 75 % of capacitance at the high current density of 1 mA/cm2. The superior performance of RGO+CNT@CMC was also attributed to its smaller resistance (0.55kW) compared with RGO@CMC (0.87 kW) and CNT@CMC (11.22 kW). All three supercapacitors showed almost no decay of capacitance after 2 000 charge/discharge cycles, and no change in performance on bending even to an angle of 180˚. The output currents of two and three supercapacitors connected in parallel are doubled and tripled respectively, while the voltages of two and three supercapacitors connected in series are doubled and tripled respectively.
The supercapacitors are flexible enough to be co-woven into a cloth or for two 40 cm long electrodes to interweave as a cloth supercapcitor (Figure 2).
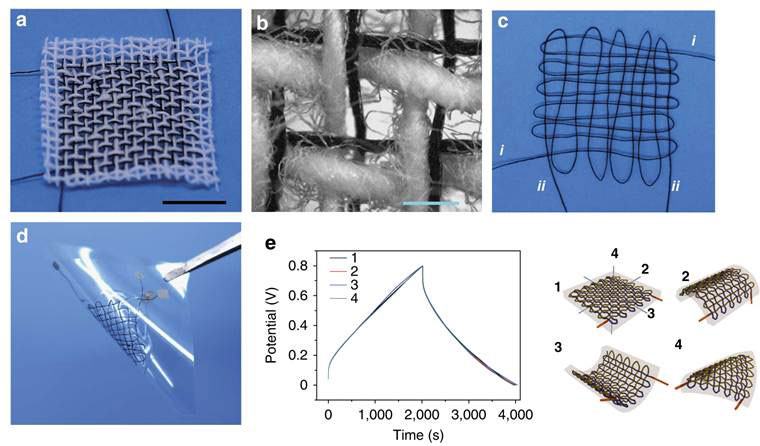
Figure 2 Woven fibre supercapacitors a, interwoven in cotton cloth; b, enlarged view of a; c, two electrodes I and ii woven into a cloth; d, the supercapacitor from c; e, charge/discharge curves of the unbent supercapacitor (1) compared with curves when variously bent (2-4)
A Ragone plot (see [6]) shows that the best performing supercapacitor has a high energy density of 3.5 mWh/cm3 but a modest power density of 0.o18 W/cm3.
The energy density E of microsupercapacitors is given by the equation E = Ccell V2/2 where Ccell is the cell capacitance, V is the operating voltage. Thus, energy density could be enhanced by high capacitance electrode material or increasing the cell voltage. One way to increase the cell voltage is by constructing asymmetric (hybrid) micro-supercapacitors with one capacitor (electrostatic)-type electrode as power source and the other battery (electrochemical)-type electrode as energy source. A team of researchers led by Yuan Chen at Nanyang University, Singapore, designed such an asymmetric fibre micro-supercapacitor [7].
Using a silica capillary column as a micro-reactor for hydrothermal synthesis, the team made the capacitative electrode by in-situ doping of reduced graphene oxide/single wall carbon nanotube (rGO/SWCNT) fibres using urea as the nitrogen precursor. For the electrochemical electrode, they used the same hydrothermal synthesis process without urea to make an undoped all-carbon fibre, and then coated the fibres with MnO2 at ambient conditions (Figure 3).
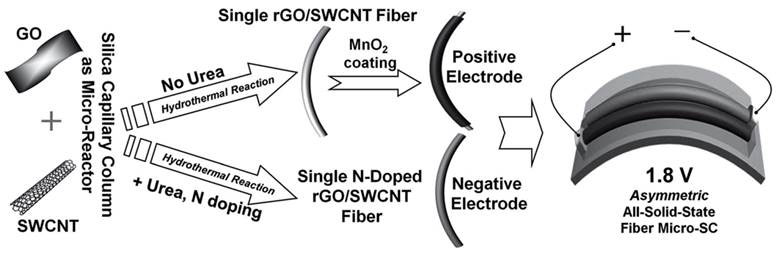
Figure 3 Fabrication of asymmetric fibre mico-supercapacitor (see text for details)
The device operates at a high-voltage of 1.8 V with a volumetric energy density of 5 mWh/cm3, higher than most solid-state micro-supercapacitors reported so far and comparable to some thin film lithium batteries.
To create the composite rGO/SWCNT, nitric acid-treated SWCNTs (~1.4 mm in diameter) and GO (several hundred nanometres to several micrometres sheets 0.7nm thick) were mixed in the hydrothermal reactor. SWCNTs have excellent electrical conductivity, but limited capacitance, whereas rGO sheets have large surface area for capacitance but poor electrical conductivity. Thus, their electrical properties would complement each other. In addition, GO is a very good surfactant for dispersing SWCNTs; SWCNTs in turn hinder the GO sheets from restacking. This makes it easy to prepare a homogeneous aqueous suspension at the optimal mass ratio for feeding into the column. The column was placed in an oven at 220 ˚C for 6 h with the two ends sealed. Afterwards, a continuous fibre was pushed into a water bath by pressurized nitrogen. The wet fibres were further dried in air for 4 h. They have a tensile strength of 88 Mpa comparable to wet-spun SWCNT fibres, and an electrical conductivity ~100 S/cm, an 8-fold increase over rGO fibres. More importantly, it has a specific length capacitance up to ~1.5 mF/cm at 5 mV/s in the 1.0 M Na2So4 electrolyte, 5-fold higher than that of rGO fibres and also much higher than those of most reported carbonaceous fibres. The high specific capacitance is attributed to the high electrical conductivity and relatively large specific surface area of 368 m2/g, as well as some oxygen-containing groups that make it more wettable. The undoped rGO/SWCNT fibres were dipped into 0.1M KMnO4/0.1M Na2 SO4 solution at room temperature for 5 to 30 minutes, resulting in different mass % of MnO2 adsorbed.
On testing, the capacitance of the MnO2-coated fibres was found to increase from 1.5 (uncoated) to 1.76, 2.36, 3.01 and 3.3 mF/cm at a scan rate of 5 mV/s at dipping times of 0, 5, 10, 20 and 30 minutes respectively. However, the rate capability of the fibres deteriorates with increase in dipping time. At 30 minutes, the fibre retained only 53 % of its capacitance when the scan rate increased from 5 to 100 mV/s.
For the capacitative electrode, the mass ratio of urea to GO and SWCNT were varied from 1:1:1 to 2:1:1 to 4:1:1. Among all the doped fibres, the 2:1:1 fibre has the largest specific length capacitance of 2.37 mF/cm at a scan rate of 5 mV. The same fibre also exhibited excellent rate capability retaining 73 % of capacitance when the scan rate increased from 5 to 100 mV/s. Nitrogen doping increases the wettability of the carbon surface and electrical conductivity of the rGO/SWCNT fibres.
The asymmetric micro-supercapacitor was fabricated using the MnO2-coated fibre (dipped for 10 minutes) as the positive electrode as its specific capacitance of 2.36 mF/cm best matched that of the 2:1:1 N-doped electrode that performed the best.
The resulting micro-supercapacitor performed as expected. The CV profiles were almost rectangular at the scan rate of 10 mV even at the high potential of up to 1.8 V (the sum of voltages of the two asymmetric electrodes). The volumetric capacitance was ~11.1F/cm3 at a current density of 25 mA/cm3 dropping to ~5.2 F/cm3 at ~ 1 000 mA/cm3, indicating good rate capability. It has a maximum volumetric energy density of ~5 mWh/cm3, about 8-fold higher than those of typical bulk supercapacitors and comparable to the thin film lithium battery. The volumetric power density was 0.929W/cm3, comparable to typical commercially available supercapacitors and about 2 orders of magnitude higher than that of thin film lithium batteries. The micro-supercapacitor retained 87 % of its initial capacity after 10 000 charge-discharge cycles, and operated normally on bending up to 120˚.
As a demonstration of its use in a self-powered nanosystem, three micro-supercapacitors were connected in series to a ZnO photodetector. It showed robust current response to the presence of UV light.
Whole new generations of consumer microelectronics and wearable electronics will be hitting the market powered by micro-supercapacitors that are paper-thin, or whisker thick, printed or spun. There is an urgent need to design for reuse and recycling now and to strictly limit the electronic and polymer wastes to be added to an already overloaded environment.
Article first published 08/04/15
Comments are now closed for this article
There are 3 comments on this article.
Mae-Wan Ho Comment left 9th April 2015 21:09:01
Robert and Gene,
You are both right about the importance of safety research, which is almost nil at the moment.
The high aspect ratio of the manufactured fibres are not the same problem as individual carbon nanotubes as these are generally micron scale and bigger.Nevertheless they can still be toxic. Similarly, the numerous liquid crystalline graphenes created have yet to be tested for toxicities, while the manufacturing processes including solvents used and handling of nanoparticles definitely need more attention.
And finally, the waste generated will be deadly to all creatures that swallow them or get entangled in them.
R.M. Davidson M.D. Ph.D. Comment left 9th April 2015 21:09:31
The high aspect-ratio of a number of these nanoparticles might pose a health risk if inhaled. Moreover, the liquid-crystalline water environment, in vivo, might be disrupted. Safety should be a major concern, going forward.
Gene Sperling Comment left 9th April 2015 17:05:17
What has been the testing used to prove safety?