Recombinant spider silk can be produced safely and effectively in contained facilities from genetically engineered bacteria and silk worms, provided containment is strictly implemented Dr. Mae-Wan Ho
The potential applications of spider silk are legion on account of its unique combination of mechanical properties, not least the shimmering beauty of the natural fibre (see [1] In Praise of Spiders and Spider Silk, SiS 54), which is stronger than steel yet flexible and extensible, making it the toughest material available, hence the bulletproof vest of the headlines (see [2] Unspinning the Web of the Spider Goat, SiS 54). Apart from providing new fibres for textiles for a multitude of purposes, spider silk is ideal for use as surgical suture, as scaffolds for bone and skin in culture for tissue replacement, as drug delivery capsules, for solid states devices, biosensing films [3], parachute cords, and composite materials in aircrafts [4].
But as spiders are very difficult to farm, a great deal of effort has gone into producing spider silk by genetic engineering.
There are major challenges in producing spider silk through genetic engineering. First, the repetitive modules of the spider silk gene [1] tends to become truncated in the transgenic host through recombination between repeated modules of the same gene. Second, the high content of glycine (43-45 %) puts an unusual burden on the translation machinery of the host cell [3]. And even after the perfect silk protein is produced, the biggest hurdle of all is to spin a fibre that’s as good as the one the spider spins naturally with the greatest of ease.
The repetitive nature of the protein core, nevertheless, offers opportunities of creating synthetic genes that could modify and tailor the mechanical properties of the silk for specific applications.
Among the most successful attempts so far is the production of spider silk in genetically modified E. coli bacteria.
A group of researchers led by San Yup Lee at Korea Advanced Institute of Science and Technology in Daejeon have succeeded in producing the largest spider silk proteins in E. coli. A record-setting 284.9 kDa recombinant silk protein of Nephila clavipes (a Golden Orb spider species) was produced and spun into a fibre displaying mechanical properties ‘comparable’ to those of the native silk. The glycine-rich protein was favourably expressed in a metabolically engineered strain with an enhanced pool of glycyl-tRNA, the transfer RNA of glycine charged with the amino acid and ready to be added to the growing spider silk protein chain.
The large protein produced was important, as smaller proteins gave inferior silk fibres, and the enrichment of glycyl-tRNA boosted the production of silk proteins to high levels.
The researchers used synthetic versions of the major ampullate spidroin 1 (MaSp1) [1] containing different number of repeat modules from 32 to 96, with predicted molecular weights of 100.7 to 284.9 kDa, the largest being similar in molecular weight to the natural MaSp2 found in the spider’s silk gland.
To find out how silk gene expression affects the host proteins, the researchers performed proteomic analysis, which gives a profile of the level of expression of all the proteins in the cell. Many stress response proteins were upregulated. Importantly, the enzyme that synthesizes glycine - serine hydroxymethyltransferase (GlyA) - and the b-subunit (GlyS) of the enzyme that synthesizes glycyl-tRNA were both upregulated, suggesting an increased demand for glycyl-RNA in expressing the glycine-rich protein.
An increase in the gene dosage of glycyl-tRNA synthase did not enhance silk production. So it was decided to increase the glycyl-tRNA pool by overexpressing the genes encoding the tRNAs in a plasmid. This resulted in higher expression of the four larger spider silk proteins (48-96-mer), and in all instances, enhanced cell growth by 30-50 %. The expression levels of the top two largest proteins (80-96-mer) were further increased by duplicating the glycyl-tRNA expression cassette. In addition, the glycine biosynthetic enzyme, GlyA was over-expressed, which further boosted the levels of the three largest proteins. The two modifications acted synergistically; together, they allowed 10 to 35-fold higher production of the three largest spider silk proteins.
The bacterial strains could be grown to high cell densities of >42 g/l, with maximum silk protein concentrations estimated in the range of 0.5 to 2.7 g/l (minimum estimates).
The cells were collected from 1 litre of culture, and lysed (burst open). Acid was added to the suspension, which got rid of most of the contaminating cell proteins, as silk proteins are highly positively charged, and hence acid-soluble. Fractional ammonium sulphate precipitation further enriched and purified the silk protein. Further steps resulted in 1.2 g of the silk protein with a purity of about 90 %.
Fibres were spun from all the different proteins, including a 16-mer 54.6 kDa protein, under the same spinning and drawing conditions after dissolving in hexafluoroisopropanol at a concentration of 20 % (w/v).
The mechanical properties of a fibre [1] are best described by the shape of a stress-strain curve when the fibre is stretched. The maximum height of the stress-strain curve is called tenacity, the amount of stress that a fibre can take before being torn apart. The furthest horizontal extent of the stress-strain curve is breaking strain, representing the extensibility of the fibre. The ratio of stress to strain is Young’s modulus, which is derived from the slope of the curve as a measure of the fibre’s stiffness. The 96-mer fibre exhibited a tenacity of 508 + 108 MPa and elongation of 15 + 5%, which are comparable to the values reported for native N. clavipes dragline silk (740–1 200 MPa; 18–27%). Notably, Young’s modulus of the 96-mer fibre was 21 + 4 GPa, twice that of the native dragline silk (11–14 GPa). Previously, a 60 kDa recombinant dragline silk protein of the spider Araneus diadematus was spun into a fibre with average tenacity ~ 260 MPa, 4.2-fold below that of the native silk at 1 100 MPa, possibly due to the small size of the recombinant protein. The tenacity of the 96-mer fibre (508 + 108 MPa) is the highest ever reported for recombinant spider silk proteins (see Figure 1).
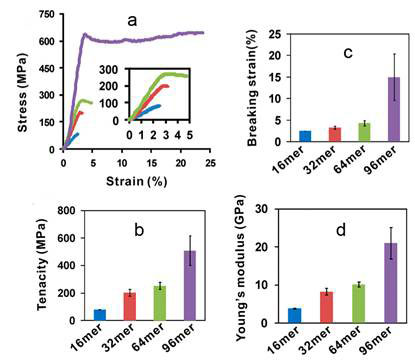
Figure 1 Analysis of fibres spun from recombinant silk proteins; a, Typical stress strain cruves of 16-mer (blue), 32-mer (red), 64-mer (green) and 96-mer (purple) fibres, from Xia et al, 2011 [4]
Scanning electron microscopy analysis of fibre surface revealed fibrillar structure in all the fibres, as for native spider dragline silk. Analysis of fractured fibres showed many irregular voids in the shorter fibres, whereas those defects were absent in the 64- and 96-mer fibres, and suggests why longer silk proteins make stronger fibres (Figure 2).
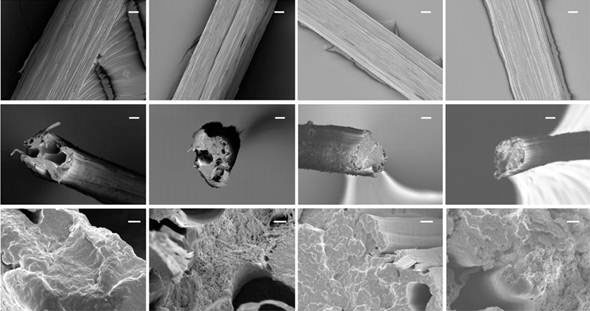
Figure 2 Scanning electron micrographs of the fibres spun from (left to right) 16-mer, 32-mer, 64-mer and 96-mer proteins; top surface of fibres, middle, and bottom, fractured surface, scale bar top and middle 10 mm, bottom 1 mm, from Xia et al , 2011 [4]
The E. coli system has the great advantage that the bacterial genome is well-characterized and understood, and can be manipulated quite precisely. It holds considerable promise for producing good quality spider silk efficiently and safely under strictly contained conditions. One disadvantage is that it cannot produce the silk fibre in a ready-spun form.
The silkworm Bombyx mori is another promising host for spider silk production. A major limitation for producing good quality spider silk is the spinning process that makes fibres from the silk proteins. Transgenic silkworms can potentially produce recombinant spider silk fibres ready-spun, provided that the spider silk protein is targeted to the worm’s silk gland with tissue-specific promoter.
A team of researchers led by Donald Jarvis at the University of Wyoming, Laramie, in the United States used the piggyBac insect vectors to transform silkworms with a synthetic spider silk gene containing 14 repeats of a module containing (A)8.(GPGGA)8, where A stands for alanine, G, for glycine, P for proline. The repeats were joined to the C- and N- termini peptides of the silkworm fibroin heavy chain gene, and placed under the control of the fibroin heavy chain promoter and enhancer sequences. The construct was designed to produce worm-spider hybrid silk that can be isolated from the worm’s cocoon. In another version of the vector, the spider silk sequence was also joined to a green fluorescent protein gene to aid in identifying transgenic silks and worms.
Transgenic silkworms were produced, and those with the green fluorescent protein readily identifiable by visual inspection of the cocoons as well as the silk glands. The chimeric silkworm/spider silk was analysed and determined to be about 2 to 5 % spider silk. The hybrid silk proteins were about 100 to 130 k Da.
Despite the low levels of spider silk protein incorporated into the chimeric fibres, they were significantly tougher than the silkworm fibres, and as tough as the native dragline silk, even though not as strong.
Like the E. coli system, the transgenic silkworms can be kept under strictly contained conditions, and holds much promise for farming spider silk or hybrid spider silkworm silk with the requisite properties. It is important to note that the piggyBac insect vector is a transposon, and has a tendency to remobilize and spread by horizontal gene transfer (see [6, 7] Regulation of Transgenic Insects Highly Inadequate & Unsafe, and Transgenic Mosquitoes Not a Solution, SiS 54). That is why transgenic silkworms created using this method must be reared under strictly contained conditions.
Recombinant spider silk can be produced safely and effectively under strictly confined conditions in transgenic E. coli and transgenic silkworms. There is no reason to continue with production in transgenic goats, a system that has remained characterized, and is neither ethical nor safe [2].
Article first published 19/03/12
Comments are now closed for this article