The WHO expert panel reclassified glyphosate as ‘probably carcinogenic’ more than 40 years after it was brought to market, but the range of available evidence is sufficient to classify it definitely carcinogenic. Glyphosate’s carcinogenic potential has been known to Monsanto and the US EPA from long term animal experiments since the early 1980s but repeatedly dismissed. This has resulted in two decades of people and planet being poisoned by glyphosate herbicides on a misclassification of ‘noncarcinogenic’ that has allowed the manufacturer to claim it is ‘safe’ and perpetrating many other falsehoods to promote its ubiquitous and liberal use Dr. Mae-Wan Ho and Prof Peter T. Saunders
A fully referenced version of this article is included in the I-SIS report Banishing Glyphosate

The International Agency for Research on Cancer (IARC), which is part of the World Health Organization (WHO), has released the results of its year-long assessment of five organophosphate insecticides and herbicides. In this, the 112th study into potentially carcinogenic agents, it has reclassified glyphosate in Group 2A ‘probably carcinogenic to humans’ [1, 2]. This category is used [1] “when there is limited evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals.” Previously, the US Environment Protection Agency (EPA), which last reviewed glyphosate in 1993, classified glyphosate in Group E ‘noncarcinogenic’ [3]. Similarly, a 2013 review by the German Institute for Risk Assessment (BfR) conducted on behalf of the European Union did not recommend a carcinogen classification of either 2A or 2B (‘possibly carcinogenic’); though that review is mired in controversy, having been largely conducted by a consortium of chemical companies including Monsanto [4] (Scandal of Glyphosate Re-assessment in Europe, SiS 63). Monsanto has called on IARC to retract its assessment [5]. But Aaron Blair, scientist emeritus at the National Cancer Institute who chaired the 17 member Working Group of the IARC that carried out the assessment, robustly defended the decision, saying it is “appropriately based on current science” [6]. The IARC experts in the Working Group were selected for their expertise and most importantly, the absence of real or apparent conflicts of interest. Following the protocol required by the IARC, the Working Group considered only “reports that have been published or accepted for publication in the openly available scientific literature” as well as “data from governmental reports that are publicly available”.
Notably, Blair told a journalist there were good grounds to declare that glyphosate definitely causes cancer [7]. But “the epidemiologic data was a little noisy.” While several studies suggested a link, another study in the US of farmers in Iowa and North Carolina did not. There had been a similar inconsistency in epidemiological studies of benzene now universally acknowledged as a carcinogen, Blair added.
What Blair did not mention, for example, was that crucial evidence of carcinogenicity in animal experiments had existed at least since 1981 but successively dismissed as documented in EPA’s own archives (see below). In the meantime, US government data show steep rise in dozens of chronic diseases including cancers closely tracking the rapid increase in glyphosate use and the adoption of genetically modified (GM) crops, of which USA is the top producer by far [8]. In Argentina, the third producer of GM crops, where the use of pesticides including glyphosate has increased more than 8.5-fold since the introduction of GM crops 20 years ago, non-government organizations and doctors have been documenting rising incidences of cancers and birth defects among farmers and their families and others exposed to glyphosate spraying (see Chapter 3 of [9] Banishing Glyphosate, I-SIS special report). Taking these and other additional findings into proper account would surely have been sufficient to classify glyphosate as a carcinogen.
Asked to comment on the IARC’s reclassification, Fernando Manas, a member of the Genetics and Environmental Mutagenesis (GEMA) Group at the National University of Río Cuarto in Córdoba, Argentina, who has investigated the effect of agrochemicals for the past 9 years, confirmed the link between glyphosate and genetic damage, which leads to cancer and a higher risk of spontaneous abortions and birth defects in the new born, and said that the classification by IARC-WHO is a consequence of the growing scientific evidence generated by independent investigators. Furthermore, he pointed out that [10] this evidence, which has been deliberately ignored until now, means that “millions of gallons of herbicide with carcinogenic potential have been used according to regulations designed for a virtually harmless substance.” For two decades, entire populations were “subjected” to chronic pesticide exposures “based on criteria developed by the same companies that produce and market” agrochemicals.
The IARC Monograph Volume 112 detailing the deliberations on all five organophosphate pesticides is yet to be published in full, but the part dealing with glyphosate is available, running to 92 pages [11]. It concluded that there is
The overall evaluation places glyphosate in Group 2A, probably carcinogenic to humans. In addition, the IARC Working Group noted other relevant data supporting the classification.
Genotoxicity and oxidative stress are both recognized as key characteristics of known human carcinogens.
The IARC report on glyphosate is comprehensive, dealing with many other aspects of its toxicity. In this review, we shall limit ourselves to the key aspects of the evidence relating to its carcinogenic potential, as outlined above, and to include relevant findings not covered by the IARC report.
The Working Group identified 7 reports from the Agricultural Health Study (AHS), a large prospective cohort study of farmers and pesticide applicators in North Carolina and Iowa - people most likely to be exposed to pesticides [12]. (For explanations of terms see Box 1). The AHS cohort, a pooled analysis of the case-control studies in the Midwest USA, and the cross-Canada study were considered key investigations on account of their relatively large size. Reports from two or more independent studies were available for non-Hodgkin lymphoma (NHL), multiple myeloma, Hodgkin lymphoma, glioma, and prostate cancer. For other cancer sites, only one study was available for evaluation.
Box 1
Cohort study A cohort is a group of people who share a common attribute or experience. A cohort study follows over a period of time such a group of people who do not have the disease and uses correlations to determine the absolute risk of contracting the disease (modified from [13])
Case-control study A study that compares patients who have a disease (cases) with patients who do not have the disease from the same population (controls), and looks back retrospectively to compare how frequently the exposure to a risk factor is present in each group to determine the relationship between the risk factor and the disease (modified from [14])
Relative risk (RR) Ratio of the probability of disease occurring in exposed group to the probability of disease occurring in a non-exposed control group, where probability in each group is defined as number of diseased/total number in group (modified from [15])
Odds ratio (OR) The odds of disease occurring in exposed group to the odds of disease occurring in the non-exposed group, where the odds in each group is calculated as number of diseased/number of healthy (modified from [15])
95 % confidence interval (CI) A confidence interval is the range within which the data indicate a parameter - such as the population mean - is to fall (see for example, [16]); most studies use 95 %, which correspond to significance at the 5 % level.
Glyphosate exposure and NHL
Two large case-control studies of NHL from
Canada and the USA, and two case-control studies from Sweden reported
statistically significant increased risks of NHL with glyphosate exposure.
The Canadian multicentre population-based case-control study on specific pesticide exposure and NHL published in 2001 involved 517 cases and 1 506 controls among men of 6 Canadian provinces [17]. Odds ratios (ORs) of 1.26 (95 % CI 0.87-1.80; 51 exposed cases adjusted for age and province) and 1.20 (95 % CI 0.83-1.74, adjusted for age, province and high-risk exposures) were found for exposure to glyphosate. Participants with >2 days of exposure per year had an OR of 2.12 (95 % CI 1.20-3.73, 23 exposed cases) compared with those with < 2 days of exposure.
The population-based case-control study among men in 6 Canadian provinces between 1991 and 1994 also investigated association between lifetime use of pesticides and multiple myeloma (a subtype of NHL) [18]. Data from 342 cases of multiple myeloma and 1 357 controls were obtained for ever-use of pesticides, number of pesticides used, and days per year of pesticide use. The OR for ever use of glyphosate was 1.19 (95 % CI 0.76-1.87; 32 cases). When the analysis was done for level of exposure, no association was found for light users <2days per year of exposure; while the OR in heavier users (> 2 days of exposure per year) was 2.04 (95 % CI 0.98-4.23, 12 exposed cases).
The US study published in 2003 [19] used pooled data from three case-control studies of NHL conducted in the 1980s in Nebraska, Kansas, and in Iowa and Minnesota. The study population included 870 cases and 2 569 controls; another 650 cases and 1933 controls were included for the analysis of 47 pesticides to control for potential confounding by other pesticides. Based on 36 cases exposed, the OR for association between glyphosate exposure and NHL were 2.1 (95 % CI 1.1-1.4) in the logistic regression analysis and 1.6 (95 % CI 0.9-2.8) in the hierarchical regression analysis, where adjusted estimates were based on prior distributions for the pesticide effects, which provides more conservative estimates than logistic regression.
The incidence of 12 cancers – lung, melanoma, multiple myeloma, NHL, oral cavity, colon, rectum, pancreas, kidney, bladder, prostate and leukaemia - was investigated among the 57 311 glyphosate-exposed pesticide applicators in the AHS study [20]. Glyphosate exposure was not associated with all cancers combined, or with most of the cancer subtypes studied. There was a suggested association with multiple myeloma (a subtype of NHL). The RR was 1.1 when adjusted for age (95 % CI 0.9-1.2, 32 cases), and 2.6 (95 CI 0.7-9.4) when adjusted for multiple confounders: age, smoking, other pesticides, alcohol consumption, family history of cancer and education). In the analysis of cumulative exposure days and intensity weighted exposure days, the RRs were around 2.0 in the highest third of the exposed subjects. The association between multiple myeloma and exposure to glyphosate only appear within the subgroup for which complete data were available on all the covariates, even without any adjustment. A re-analysis of these data [21] confirmed that the excess risk of multiple myeloma was present only in the subset with no missing information (22 cases in the restricted data set). The AHS sought information on the use of 50 pesticides [12] and it has been demonstrated that misclassification of pesticide exposure would bias relative risk estimates in the AHS towards the null and diminish the power of the study [22].
Successive studies in Sweden since 1998 reported association of NHL with glyphosate use, but the numbers were small (reviewed in [11, pp. 26-27]). A pooled analysis of two case-control studies one on NHL and another on hairy cell leukaemia (a subtype of NHL) based on 515 cases and 1141 controls published in 2002 [23] reported increased risk for exposure to glyphosate. The OR was 3.04 (95 % CI 1.08-8.52, 8 exposed cases) in the univariate and 1.85 (95 % CI 0.55-6.02) in a multivariate analysis that considered study, study area, and vital status. A population-based case-control study of exposure to pesticides as a risk factor for NHL published in 2008 included men and women aged 18-74 years living in Sweden from 1 December 1999 to 30 April 2002, giving a total of 910 cases and 1 016 controls matched for age and sex [24]. The OR for exposure to glyphosate was 2.02 (95 % CI 1.10-3.71) in a univariate analysis and 1.51 (95 % CI 0.77-2.94) in a multivariable analysis. When exposure for more than 10 days per year was considered, the OR was 2.36 (95 % CI 1.10-3.71). The association of glyphosate exposure with lymphoma subtypes was also found; for B-cell lymphoma, OR 1.87 (95 % CI 0.998-3.51) and subcategory of small lymphocytic lymphoma/ chronic lymphocytic leukaemia, OR 3.35 (95 % CI 1.42-7.89, not adjusted for other pesticides). (NHLs are a heterogeneous group of more than 20 B- and T-cell lymphomas affecting the immune system/lymphatic system and arising primarily in the lymph nodes [25].)
A hospital-based case-control study was conducted at 6 centres in France between 2000 and 2004 of cases with a diagnosis of lymphoid neoplasm aged 20-75 and controls recruited in the same hospital [26]. The analysis included 491 cases (244 cases of NHL, 87 cases of Hodgkin lymphoma, 104 lymphoproliferative syndrome, and 6 cases of multiple myeloma) and 456 age- and sex-matched controls. ORs associated with any exposure to glyphosate were 1.2 (95 % CI 0.6-2.1; 27 cases) for all lymphoid neoplasmas combined, 2.4 (95 % CI 0.8-7.3) for multiple myeloma, and 1.7 (95 % 0.6-5.0; 6 cases) for Hodgkin lymphoma, after adjusting for age, centre, and socioeconomic category.
A pooled analysis of case-control studies conducted in 6 European countries in 1998-2004 – Czech Republic, France, Germany, Ireland, Italy, and Spain - involved 2 348 cases of lymphoma and 2 462 controls [27]. Lymphoma overall and B-cell lymphoma were not associated with any class of the investigated pesticides, while the risk of chronic lymphocytic leukaemia was elevated among those ever exposed to inorganic and organic pesticides. The ORs for glyphosate exposure and B-cell lymphoma was 3.1 (95 % CI 0.6-17.1, 4 exposed cases and 2 exposed controls).
A systematic review and meta-analysis of NHL and occupational exposure to agricultural pesticides [28] for which 6 previous studies were included [17, 19, 20, 23, 24, 26] yielded a meta risk ratio of 1.5 (95 % CI 1.1-2.0). The Working Group noted that the most fully adjusted risk estimates from [23, 24] were not used. After considering the adjusted estimates of these two Swedish studies, the Working Group estimated a meta risk-ratio of 1.2 (95 % CI 1.03-1.65) (see [11, p.30]).
Glyphosate exposure and other cancer
sites
A case-control analysis nested in the AHS
examined associations between pesticide use and cancer of the pancreas included
93 incident cases (64 applicators, 29 spouses) and 82 503 cancer-free controls
The OR for ever versus never exposure to glyphosate was 1.1 (95 % CI
0.6-7.55; 55 exposed cases), while the OR for the highest category of level of
intensity-weighted lifetime days was 1.2 (95 % CI 0.6-2.6, 19 exposed cases)
[29].
An investigation on the relationship between agricultural pesticide exposure and incidence of cancer of the colorectum in the AHS included 56 813 pesticide applicators with no prior history of cancer of the colorectum, and 305 incidents of cancer of the colorectum (colon 212, rectum, 93) diagnosed during the study period 1993-2002 [30]. Most of the 50 pesticides studied were not associated with risk of colorectal cancer. The relative risks with exposure to glyphosate were 1.2 (95 % CI 0.9-1.6), 1.0 (95 % CI 0.7-1.5) and 1.6 (95 % CI0.9-2.9) for cancers of the colorectum, colon, and rectum respectively.
A case-control study of 1 516 patients with prostate cancer in British Columbia, Canada, from 1983 to 1990 and 4 994 age-matched controls with cancers at all other cancer sites excluding lung and unknown primary site reported OR for glyphosate exposure 1.36 (95 % CI 0.83-2.25, 60 cases) [31].
No association with glyphosate exposure was found in the AHS for childhood cancer, breast cancer among farmers’ wives, prostate cancer, cutaneous melanoma (each represented by a single study, reviewed in [11]). No association was found for in case-control studies for glyphosate exposure and adenocarcinomas of the oesophagus and stomach (one study), glioma (three studies), or soft tissue sarcoma (one study) as reviewed in [11].
In summary, there is evidence that glyphosate exposure is associated with increased risk of non-Hodgkin’s lymphoma from several large studies as well as smaller studies. In addition, single studies have found non-significantly increased RRs or ORs for glyphosate exposure and several cancer sites.
Glyphosate contamination ubiquitous
in the environment
Glyphosate herbicides have been marketed
since the 1970s, but the steep rise in their use began with the commercial
release of GM glyphosate-tolerant crops 30 years ago, and they rapidly become
the world’s top selling herbicides. Currently,
85 % of GM crops planted globally are herbicide-tolerant, with glyphosate-tolerant
crops making up the vast majority of those planted [32]. In the USA, the
largest producer of GM crops, 93 % of soybean, 85 % of cotton, and 85 % of
maize crops are glyphosate-tolerant [33]. In recent years, the use of
glyphosate herbicides has expanded to include weed control in residential and
commercial areas and as desiccant to aid in harvesting a wide range of
conventional non-GM crops [34]. The global glyphosate market demand in 2012 was
718 600 tonnes [35], with GM crops accounting for 45.2
% of the total demand, and glyphosate ~25 % of the global pesticide market
[36]. In the USA alone, overall pesticide use increased by an estimated 183
million kilograms (404 million pounds) in the first 16 years of GM crops
between 1996 and 2011 [37]; and glyphosate is estimated to account for ~40 % of
all pesticide use (by weight of active ingredient) from figures provided by the
US EPA in 2007 [38]. Glyphosate and glyphosate residues have contaminated the
entire environment, air, soil, water, urban, suburban, and rural, representing
an enormous increase in the pesticide burden on global health.
A compilation representing the largest and most comprehensive assessment of the environmental occurrence of glyphosate and AMPA in the US conducted to-date summarises the results of 3 732 water and sediment and 1 018 quality assurance samples collected between 2001 and 2010 from 38 states and the District of Columbia [39]. The results indicate that glyphosate and AMPA are detected frequently together, that they are mobile and occur widely in the environment. Overall, glyphosate was detected in 39.4 % of samples (median < 0.2, maximum 476 μg/L or kg in soil and sediment), and AMPA in 55.0 % (median 0.05, maximum 397 μg/L or kg in ditches and drains). Glyphosate and AMPA were detected frequently in soils and sediment (91.1 % and 93.3 % respectively), ditches and drains (70.9 % and 80.7 % respectively), precipitation (70.6 % and 71.8 % respectively), rivers (53.1 % and 89,3 % respectively) and streams (52.5 % and 55.0 % respectively, and less frequently in lakes, ponds, and wetlands (33.7 % and 29.8 % respectively), soil water (34.5 % and 65.5 % respectively, and groundwater (5.8 % and 14.3 % respectively).
Glyphosate builds up and leaches from
soil
Glyphosate is a polar amphoteric compound
that binds strongly to soils but is also very soluble in water. It has a soil
half-life ranging from 2 to 215 days, and an aquatic half-life of 2 to 91 days.
Glyphosate degrades in the environment primarily by microbial action to AMPA,
which is also very water soluble, and degrades more slowly than glyphosate.
AMPA has a soil half-life of 60-240 days and an aquatic half-life comparable to
that of glyphosate. AMPA ultimately degrades to inorganic phosphate, ammonium
and CO2, adding phosphate pollution to aquatic systems (reviewed in [39]). Recent
samplings in Argentina showed glyphosate levels in rain water averaging 6.5 μg/L and as high
as 67 μg/L, more than 20 times the level in the USA. In Spain all 11
groundwater sites sampled were positive for glyphosate despite it being a
region free from glyphosate-tolerant GM crop cultivation (reviewed in Chapter 1
of Banishing Glyphosate [9]). Thus both
glyphosate and its main metabolite AMPA are long-lasting in the environment,
and leach easily into water; this is contrary to claims by the manufacturer,
which has been repeatedly prosecuted for false advertising that Roundup is
“biodegradable”, “won’t build up in the soil”, “no leaching”, and “less toxic
to rats than table salt”, “‘practically non-toxic’” to mammals, birds and fish”
[40, 41].
Glyphosate bioaccumulates
In the IARC report on glyphosate, it is
stated that [11, p. 45]: “Overall, systemically absorbed glyphosate is not
metabolized efficiently, and is mainly excreted unchanged in the urine.” This
has been shown to be false, as AMPA has been detected frequently in human
urine, and glyphosate in human mother’s milk and in animal tissues (reviewed in
Chapter 1 of Banishing Glyphosate [9]).
A study commissioned by Friends of the Earth Europe analysed 182 volunteers across 18 EU countries found that 80 (43.9 %) have glyphosate, with a mean of 0.21 μg/L and a maximum of 1.82 μg/L. AMPA was present in 65 (35.71 %), with a mean of 0.18 μg/L and a maximum of 2.63 μg/L. In the US, urine samples show concentrations 8 times those in Europe. The analysis, commissioned by Moms Across America, also tested 10 mothers’ breast milk, which came up positive for glyphosate with levels ranging from 76 to 166 µg/L, higher than those in urine, and 760 to 1600 times higher than the European Drinking Water Directive allowed levels for individual pesticides, falling within the range of concentrations at which developmental toxicity has been observed in animal studies. A second study on breast milk commissioned by the Green Party was performed in Germany, where far fewer GM crops are consumed, and glyphosate levels ranged from 0.210-0.432 µg/L, well above the EU drinking water limit of 0.1 µg/L.
In a peer-reviewed study published in 2014 [42], not included in the IARC assessment, glyphosate was detected in human, cow, rabbit and hare urine as well as in tissues of cows. Samples were collected from Germany (except for urine from Danish cows) as follows: urine of cows from conventional farms in (N=343), urine from cows kept in GM free areas (N=32); organs from slaughtered cows from conventional husbandry: gut wall (N=32), liver (N=4), kidney (N=26), lung (N=23) and muscle (N=6); urine from Danish cows (N = 242); urine from 192 hares and 77 fattening rabbits; human urine from 99 on conventional diet and 41 on organic diet; and further human urine samples from 102 healthy subjects and 199 chronically ill subject. A two-way analysis of variance followed by unpaired Student’s t-tests was used to identify significant differences between means. The results are presented in Figure 1 (unfortunately, the authors chose not to tabulate the numerical values). As can be seen, urine from German cows had on average significantly less glyphosate than urine from cows in Denmark (p<0.0001); cows kept in GM free regions had significantly lower concentration of glyphosate in their urine than cows kept on conventional farms (p<0.001); glyphosate was detected in all the organs of slaughtered cows with no significant difference between the means; hares showed significantly lower glyphosate residues in urine than in fattening rabbits (p<0.0001); humans on conventional diet had significantly higher glyphosate levels than those on organic diet (p<0.0002), and healthy humans had significantly lower glyphosate levels in urine than those with chronic disease (p<0.03). The results clearly show that glyphosate and glyphosate residues could be ingested in food and feed and drinking water (or indeed absorbed through the air or through the skin, see later) and excreted in urine. Furthermore, they can accumulate in all tissues, and at levels known to promote the growth of cancer cells in vitro (see below).
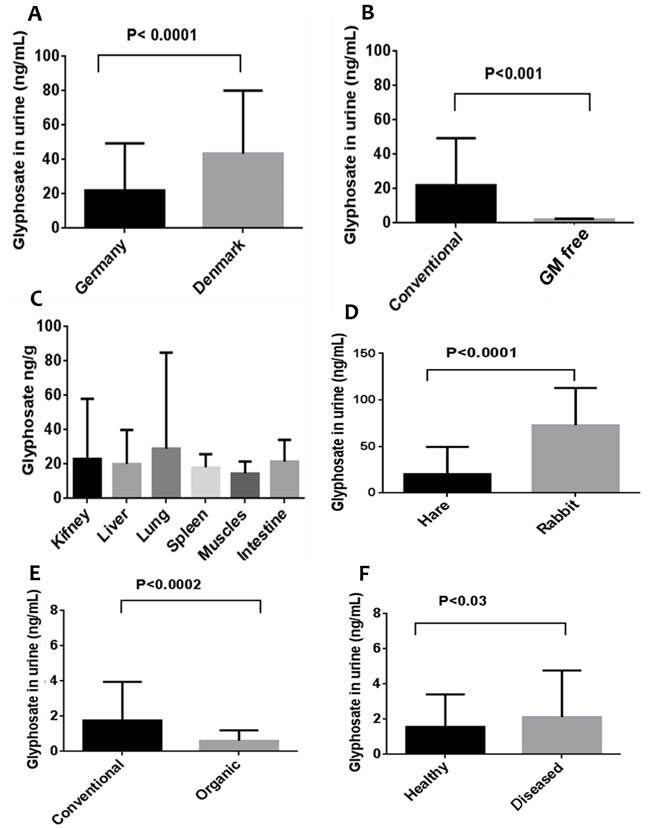
Figure
1 Glyphosate residues in urine and animal tissues: A, urine of cows in
Germany and in Denmark; B, urine of cows from conventional and GM free farms;
C, levels in different organs and tissues from cattle obtained in a slaughter
house; E, urine from humans on conventional and organic diets; F, urine from
healthy humans and those chronically disease
(redrawn
from [42])
Marked deterioration on public health
tracks glyphosate and GM crops increase
Although there has been no official health
monitoring for glyphosate or GM crops as such, it is possible to examine the
health status of countries that have seen the steepest rise in glyphosate use
before and after the introduction of GM crops when the rapid increase in
glyphosate use began. Plotting the best available government data year to year
from the US Centers for Disease Control for the incidence of diseases in the
country, and the Department of Agriculture for the GM crops grown and
glyphosate herbicides used, Swanson et al [8] showed increases in the incidence
of dozens of diseases including six cancers closely tracking the increases in
GM crops and glyphosate usage. Figure 2 shows the incidences of liver and
thyroid cancers, the former with a distinct pre-1990 trend, the latter without.
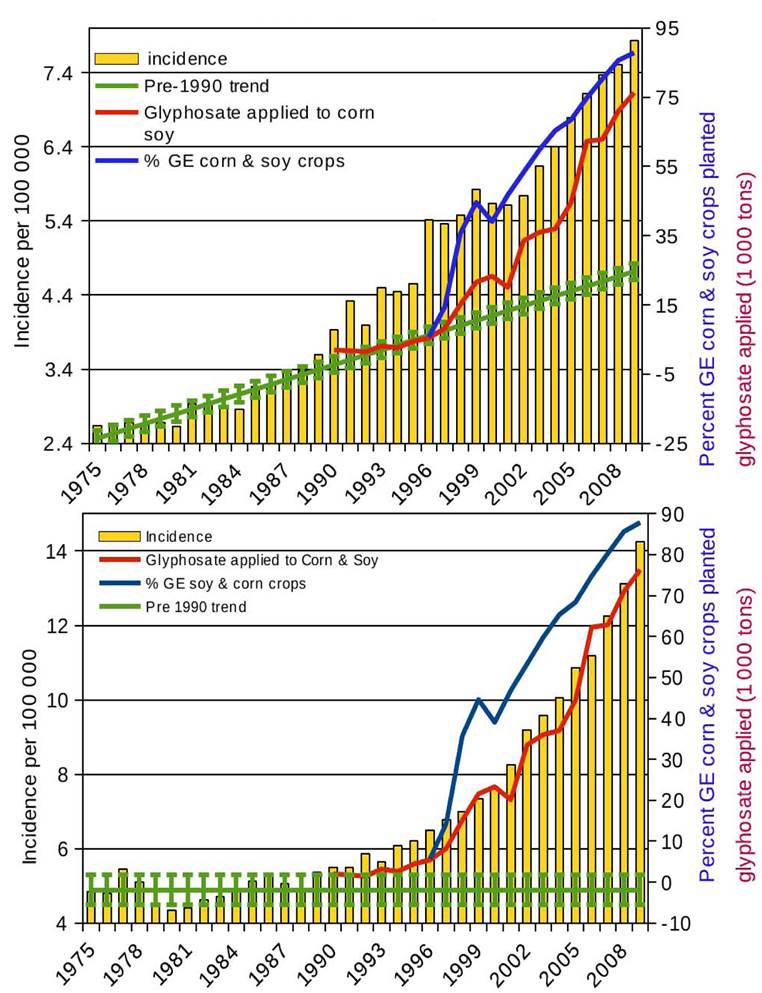
Figure 2 Incidence of liver cancer (top) and thyroid cancer (bottom) closely tracking increases in glyphosate use and GM corn and soy crop planted (redrawn from [8])
When the Pearson correlation coefficients between the incidence of 22 diseases and the amount of glyphosate used and the percentage of GM maize and soy planted respectively, most of the 44 coefficients are greater than 0.91 and none of them fall below 0.81 (see Table 1) (from Chapter 2 of Banishing Glyphosate [9]).
Table 1: Pearson correlation coefficients between the incidence in the US of 22 chronic diseases since 1995 and (a) the amount of glyphosate applied to maize and soy (b) the percentage of maize and soy planted that was GM
| Condition | Glyphosate use | %GM |
| Hypertension | 0.923 | 0.961 |
| Stroke | 0.925 | 0.983 |
| Diabetes prevalence | 0.971 | 0.983 |
| Diabetes incidence | 0.935 | 0.955 |
| Obesity | 0.962 | 0.962 |
| Lipoprotein metabolism disorder | 0.973 | 0.955 |
| Alzheimer’s | 0.917 | 0.937 |
| Senile dementia | 0.994 | 0.918 |
| Parkinson’s | 0.875 | 0.952 |
| Multiple sclerosis | 0.828 | 0.876 |
| Autism | 0.989 | 0.93 |
| Inflammatory bowel disease | 0.938 | 0.812 |
| Intestinal infections | 0.974 | 0.901 |
| End stage renal disease | 0.975 | 0.958 |
| Acute kidney failure | 0.978 | 0.967 |
| Thyroid cancer | 0.988 | 0.938 |
| Liver cancer | 0.960 | 0.911 |
| Bladder cancer | 0.981 | 0.945 |
| Pancreatic cancer | 0.918 | 0.841 |
| Kidney cancer | 0.973 | 0.940 |
| Myeloid leukaemia | 0.878 | 0.889 |
In Argentina, where the use of pesticides including especially glyphosate herbicides has increased more than 8.5-fold since GM crops were introduced 20 years ago [9], physicians and local governments have been documenting rapid increases in birth defects and cancers for years. At the 1st National Meeting of physicians in the crop-sprayed towns which took place in the National University of Córdoba 27-28 August 2010, an official report from the province of Chaco recorded a 4.5-fold increase in the incidence of birth defects over 12 years, from 19.1 /10 000 in 1997 to 28.1 /10 000 in 2001 and 85.3/ 10 000 in 2009 [43]. Also, the incidence of childhood cancer rose from 8.03/ 100 000 in 1991 to 11.2/100 000 in 2001 and 15.7/100 000 in 2007. A second report released by the Ministry of Health in Córdoba, entitled “Report on cancer in Córdoba 2004-2009” based on analysis of deaths from cancerous tumours in the province shows that the highest rates of deaths occur in areas where GM crops and agro-chemicals are used, and they are almost double the national average [44]. The provincial average is 158 per 100 000, and in Córdoba Capital, the rate is 134.8. But four Córdoba departments are well above those rates: Marcos Juárez, 229.8; Presidente Roque Sáenz Peňa, 228.4; Union, 217.4; and San Justo, 216.8. These are the “pampa gringa”, the area of Córdoba agriculture. WHO’s latest 2012 data for Argentina show that the death rate from cancerous tumours for Argentina as a whole is 115.13, about half of that in Marcos Juárez, where glyphosate and AMPA have been detected in lakes, soils, and rainwater. Apart from the worst affected pampa gringa, the departments of Rio Cuarto, General San Martin, Celman, Tercero Arriba and General Roca, also dedicated to industrial farming, have the second highest cancerous tumour deaths ranging from 180-201 per 100 000, again well above the national average.
As described in the IARC report [11], glyphosate was tested for carcinogenicity in two studies by dietary administration on male and female mice, and in male and female rats by dietary administration in 5 studies and by drinking water in one study. The main finding was a positive trend in the incidence of renal tubule carcinoma and of renal tubule adenoma or carcinoma combined in males in one feeding study in CD-1 mice; renal tubule carcinoma being a rare tumour in this strain of mice. In the second feeding study, there was a significant positive trend in the incidence of haemangiosarcoma in male CD-1 mice. For the five feeding studies in rats, two in the Sprague-Dawley strain showed a significant increase in the incidence of pancreatic islet cell adenoma in males, one of them also showed a significant positive trend in the incidences of hepatocellular adenoma in males and of thyroid C-cell adenoma in females. Two studies, one in Sprague-Dawley rats, and one in Wistar rats found no significant increase in tumour incidence at any site, but the study on Wistar rats was considered inadequate because of the short duration of exposure. The study in Wistar rats given glyphosate in drinking found no significant increase in tumour incidence.
Many of the significant results came from animal studies submitted to the US EPA, which dismissed them in altering its initial classification of glyphosate as ‘possible carcinogen’ to ‘noncarcinogenic’.
Studies with significant results
evaluated by IARC
In the first experiment submitted to the
EPA [45], groups of 50 male and 50 female randomized CD-1 mice individually
caged were given diets containing 0, 1 000, 5 000, and 30 000 ppm of glyphosate
(99.7 % pure) ad libitum for 24 months. There was a consistent decrease
in body weight in both males and female mice at the highest dose. There was a
significant positive trend (p=0.016 in trend test) in the incidence of renal
tubule adenoma in the dosed male mice: 0/49, 1/50 1/50 (2 %) 3/50 (6 %).
Subsequent to its initial report [46], the EPA recommended that additional
renal sections should be cut and evaluated for all male mice in the control and
treated groups. The pathology report indicated the same incidence of renal
tubule adenoma as originally reported [45]. The EPA then requested that a
pathology working group (PWG) be convened to evaluate the tumours of the kidney
of the male mice treated with glyphosate, including the additional renal
sections [47]. As a result, the PWG reported that the incidence of renal tubule
adenoma was 1/49 (2 %), 0/49, 1/50 (2 %), 1/50 (2 %), and not significant by
the trend test. However, the incidence of carcinoma of the renal tubule was
0/49, 0/49, 1/50 (2 %), 2/50 (4 %); p=0.037 trend test for carcinoma. The
incidence of renal tubule adenoma or carcinoma (combined) was 1/49 (2 %), 0/49,
1/50 (2 %), 3/50 (6 %); p=0.034 trend test for combined. The Working Group
considered that the second evaluation indicated a significant increase in the
incidence of rare tumours with a dose-related trend that could be attributed to
glyphosate. It has been reported that only 1 out of 725 CD-1 male mice in a
historical database had developed renal cell tumours (1 carcinoma).
The second study on groups of 50 male and 50 female CD-1 mice was reported in the Joint FAO/WHO Meeting on Pesticide Residues (JMPR). They were given diets containing glyphosate (98.6 % pure) at concentrations adjusted weekly for the first 13 weeks, and every 4 weeks thereafter to give doses of 0, 100, 300, or 1 000 mg/kg by weight ad libitum for 104 weeks. There was no effect on survival or body weight in any of the dosed groups. There was a significant increase in the incidence of haemangiosarcoma in males, 0/50, 0/50, 0/50, 4/50 (8 %); p<0.001, Cochran-Armitage trend test. There was also a non-significant increase in the incidence of histiocytic sarcoma in the lymphoreticular/haemopoietic tissues in males, 0/50, 2/50 (4 %), 0/50, 2/50 (4 %), and in females, 0/50, 3/50 (6 %), 3/50 (6 %), 1/50 (2 %).
The EPA also provided information on a long-term study of groups of 60 males and 60 female Sprague-Dawley rats (age 8 weeks) given diets containing glyphosate (96.5 % pure) at a concentration of 0, 2 000, 8 000, or 20 000 ppm, ad libitum for 24 months [48-50]. Ten animals per group were killed after 12 months. There was no effect on survival, and no significant decrease in body weight gain in males. In females at the highest dose, body weight gain was significantly decreased starting on day 51. In males at the lowest dose, there was a significant increase in the incidence of pancreatic islet cell adenoma compared with controls: 8/57 (14 %) versus 1/58 (2 %), p < 0.05 (Fisher exact test). Additional analysis by the EPA [48] using Cochran-Armitage trend test and Fisher exact test, and excluding rats that died or were killed before week 55 gave a statistically significant higher incidence of pancreatic islet cell adenoma in males at the lowest and highest doses compared with controls (1/43, 2 %): lowest dose 8/45 (18 %, p = 0.018, pairwise test), intermediate dose, 5/49 (10 %); highest dose 7/48 (15 %, p= 0.o42; pairwise test). The range for historical controls for pancreatic islet cell adenoma reported in males at this laboratory was 1.8-8.5 %. There was also a statistically significant positive trend in the incidence of hepatocellular adenoma in males (p= 0.016) and of thyroid follicular cell adenoma in females (p=0.031).
(Note that in applying the Cochran-Armitage test, the EPA assumed a linear dose response over the entire range. They gave no justification for this; indeed they did not state it explicitly. A less drastic assumption, for example, a logistic-like response, would have reduced the p-values and yielded more significant cases.)
The EPA provided information on another long-term study with groups of 50 male and 50 female Sprague-Dawley rats given diets containing glyphosate (98.7 % pure) at a concentration of 0, 30 100, or 300 ppm (mg/kg body weight per day) ad libitum for life (up to 26 months) [48-50]. An increase in the incidence of pancreatic islet cell adenoma was reported in males at the lowest dose: controls 0/50, lowest dose 5/49 (10 %), p < 0.05, Fisher exact test, both intermediate and highest dose were 2/50 (4 %).
A study on Swiss mice (20/group) tested the carcinogenic potential of glyphosate formulation Roundup Original® (glyphosate 41 %, polyethoxylated tallowamine (poea), ~15 %) dissolved in 50 % ethanol and applied onto the shaved back skin [51].
Group 1 - untreated controls;
Group 2 - glyphosate only (25 mg/kg body weight) applied topically three times per week for 32 weeks;
Group 3 - single topical application of the tumour initiator dimethylbenz[a]anthracene (DMBA in ethanol, 52 μg/mouse), followed one week later by the tumour promoter 12-O-tetradecanoylphobol-13-acetate (TPA in acetone, 5 μg/mouse) applied topically 3 times a week for 31weeks;
Group 4 – single topical application of glyphosate (25 mg/kg body weight) followed 1 week later by TPA in acetone, 5 μg/mouse) applied topically 3 times a week for 31 weeks.
Group 5 – glyphosate (25 mg/kg body weight) applied topically three times per week for 3 weeks, followed 1 week later by TPA (in acetone, 5 μg/mouse) applied topically 3 times a week for 28 weeks;
Group 6 – single topical application of DMBA (in ethanol, 52 μg/mouse);
Group 7 – TPA (in acetone, 5 μg/mouse) applied topically 3 times a week for 32 weeks;
Group 8 – single topical application of DMBA (in ethanol, 52 μg/mouse), followed one week later by glyphosate (25 mg/kg body weight) applied topically three times per week for 31 weeks.
All mice were killed at the end of the experiment (32 weeks). Skin tumours were observed in group 3, the positive control and in group 8, DMBA + glyphosate, 8/20 p<0.05 versus group 6, DMBA only, 0/20. Thus the glyphosate formulation appears to be a tumour promoter. The Working Group decided this was an inadequate study because of the small number of animals and lack of solvent controls. What the IARC report [10] did not take into account was the substantial proteomic analysis in the rest of the paper [51] using 2-dimensional gel electrophoresis and mass spectrometry. The researchers identified 22 spots that were differentially expressed (>2 fold) on glyphosate, DMBA, and TPA application over the untreated control. Among them, 9 proteins - translation elongation factor eEF-1 alpha chain, carbonic anhydrase III, annexin II, calcyclin, fab fragment anti-VEGF antibody, peroxiredoxin-2, superoxide dismutase [Cu–Zn], stefin A3, and calgranulin-B - were common and showed similar expression pattern in glyphosate and TPA-treated mouse skin. These proteins are known to be involved in several key processes such as apoptosis and growth-inhibition, anti-oxidant responses. The up-regulation of calcyclin, calgranulin-B and down-regulation of superoxide dismutase [Cu–Zn] was further confirmed by immunoblotting. The author concluded that [51]: “Altogether, these results suggested that glyphosate has tumor promoting potential in skin carcinogenesis and its mechanism seems to be similar to TPA.”
However, as the experiments were carried out with Roundup, it remains unclear whether the cancer promoting activity is due to glyphosate or POEA or both. Experiments on human cancer cells have thrown further light on the issue (see Section 5).
An excellent review on glyphosate toxicity was written by Caroline Cox of Northwest Coalition for Alternatives to Pesticides, Eugene, Oregon in the US and published in 1995.
The author stated [52]: “It is striking that laboratory studies have identified adverse effects of glyphosate or glyphosate-containing products in all standard categories of toxicological testing.” Not only is glyphosate acutely toxic to animals including humans, animal studies feeding glyphosate for 3 months resulted in reduced weight gain, diarrhoea, and salivary gland lesions. Lifetime feeding resulted in excess growth and death of liver cells, cataracts, and lens degeneration, and increases in the frequency of thyroid, pancreas, and liver tumours. Glyphosate containing products have caused genetic damage in human blood cells, fruit flies and onion cells. Glyphosate reduced sperm counts in male rats, lengthened the oestrous cycle in female rats, increasing their foetal loss and decreasing the birth weight of their offspring. The paper also revealed two serious cases of fraud in laboratories conducting toxicology and residue testing for glyphosate and glyphosate-containing products.
On carcinogenicity, Cox wrote [52]: “The potential of glyphosate to cause cancer has been a controversial subject since the first lifetime feeding studies were analyzed in the early 1980s. The first study (1979-1981) found an increase in testicular interstitial tumors in male rats at the highest dose tested (30 mg/kg of body weight per day) [53] as well as an increase in the frequency of a thyroid cancer in females [54] [this study was not considered by the IARC]. The second study (completed in 1983) found dose-related increases in the frequency of a rare kidney tumor in male mice [55]. The most recent study (1988-1990) found an increase in the number of pancreas and liver tumors in male rats together with an increase of the same thyroid cancer found in the 1983 study in females [56].”
But the EPA explained all that away. Cox continued [52]: “All of these increases in tumor incidence are “not considered compound-related” [56] according to EPA. In each case, different reasons are given for this conclusion. For the testicular tumors, EPA accepted the interpretation of an industry pathologist who said that the incidence in treated groups (12 percent) was similar to those observed in other control (not glyphosate-fed) rat feeding studies (4.5 percent) [57]. [This is an illicit use of controls, and 12 percent is clearly well above 4.5 percent in any case.] For the thyroid cancer, EPA stated that it was not possible to consistently distinguish between cancers and tumors of this type, so that the incidences of the two should be considered together [a questionable manipulation of data]. The combined data are not statistically significant [54]. For the kidney tumors, the registrants reexamined slides of kidney tissue, finding an additional tumor in untreated mice so that statistical significance was lost. This was despite a memo from EPA’s pathologist stating that the lesion in question was not really a tumor [55] [and hence amounts to a falsification of data]. For the pancreatic tumors, EPA stated that there was no dose-related trend and no progression to malignancy [the lack of linear dose-related trend is frequently the case in endocrine disrupting chemicals]. For the liver tumors and the thyroid tumors, EPA stated that pairwise comparisons between treated and untreated animals were not statistically significant and there was no progression to malignancy [56].” (Comments between square brackets added).
EPA concluded that glyphosate should be classified as Group E [56], “evidence of non-carcinogenicity for humans.” They added that this classification “is based on the available evidence at the time of evaluation and should not be interpreted as a definitive conclusion that the agent will not be a carcinogen under any circumstances.”
The EPA authorities went against the advice of their own scientists, as Cox revealed [52]. An EPA statistician wrote in a memo concerning one of the carcinogenicity studies [56], “Viewpoint is a key issue. Our viewpoint is one of protecting the public health when we see suspicious data.” Unfortunately, EPA has not taken that viewpoint in its assessment of glyphosate's cancer-causing potential. The agency should indeed be held responsible for two decades of people and planet being subjected to chronic glyphosate exposures on a misclassification that has allowed the manufacturer to claim it is ‘safe’, and perpetrating many other falsehoods to promote its ubiquitous and liberal use.
There has been only one in vivo study on the potential of glyphosate to promote cancerous growth on skin of mice [51], which suggested from proteomic analysis that the glyphosate formulations used (Roundup) promoted cancerous growth in a similar way to a well-known cancer promoter TPA, but it remained unclear whether it was glyphosate or the adjuvant POEA or both that promoted cancer. Further studies on cancer cells showed that glyphosate is probably the main culprit.
Glyphosate
promotes growth of human cancer cells
On account of epidemiological studies
showing increased frequency of birth defects in pesticide applicators and
general population of the Red River Valley, Minnesota, a selection of 16
agrochemicals including both Roundup and glyphosate (reagent grade
monoisopropylamine salt) were investigated for their effects on the growth of
the oestrogen-dependent MCF-7 human breast cancer cells [58]. Tests were
performed in both growth media contained charcoal-dextran (CD) treated or
non-CD treated foetal bovine serum. The researchers found that both glyphosate
and its most widely used formulation, Roundup, were able to promote significant
proliferation of MCF-7 cells; and the results were similar in CD- and non-CD-
treated medium. Maximum induction of cell proliferation occurred at 2.28 μg/mL pf
glyphosate (135 + 3.5 % with CD, 130 + 7.98 % without CD) or 10 μg/ml Roundup
(126 + 5.1 % with CD, 121 + 10.3 % without CD); p<0.05 linear
regression. The data suggested that non-oestrogenic induction of cell
proliferation is involved in glyphosate and Roundup (this is corroborated by
strong evidence that glyphosate and AMPA are genotoxic and cause oxidative
stress, see later).
In a second more recent and detailed study carried out in Thailand, the researchers found that glyphosate at minute concentrations enhanced the proliferation of human hormone-dependent breast cancer T47D cells, but not hormone-independent breast cancer MDA-MB231 cells. Their detailed experiments showed that glyphosate mimics the action of oestrogen, and uses the same molecular pathways as the natural hormone to promote proliferation of the cancer cells. They also found that glyphosate had synergistic effects in enhancing breast cancer cell growth in combination with genistein, a common phytoestrogen in soybean [59].
Glyphosate at concentrations between 10-12 and 10-6 M (0.169 ng/L to 0.169 mg/L) boosted the proliferation of T47D cells by 15 to 30 %, about half as effectively as the most potent oestrogen, 17 β-estradiol (E2). The same low concentrations of glyphosate induced the activation of oestrogen response element (ERE) - a specific DNA sequence promoting gene expression with high affinity for the oestrogen receptor (ER) that binds oestrogen - thereby activating gene expression in response to oestrogen. Furthermore, this activation was inhibited by an oestrogen antagonist, ICI 182780, indicating that the estrogenic activity of glyphosate was mediated via ERs.
The highest oestrogen mimicking effect was at 10-9M or 0.169 μg/L and the effect was half that of oestrogen, the most potent growth-promoter in hormone-dependent breast cancer cells. ICI 182780, a specific inhibitor of oestrogen at 1 nM reduced the proliferative effects of both glyphosate and E2. At 10 nM it completely inhibited the growth enhancing effects of glyphosate, suggesting that glyphosate acts via the oestrogen receptor ER.
T47D-KBluc cells, with stably transfected triplet oestrogen response element (ERE) promoter–luciferase reporter gene construct, when treated with glyphosate at the concentration range of 10-12 to 10-6 M, proliferated at 5-13 fold of the controls without glyphosate or E2, less than half that induced by oestrogen.
The concentration ranges of glyphosate and genistein inducing ERE activity more than 10 fold of control are individually 10-11 to 10-9M and 10-7 to 10-5 M respectively. Glyphosate residues in soybean were found in the range of 0.1-5.6μg/g, while genistein were in the range of 0.01-1.2 mg/g. As mentioned earlier, glyphosate concentrations in human urine could be 1.8 x 10-8 to 1.4 x10-6 M. Using these concentrations as a guide, the interaction range between the two oestrogenic mimics were set at genistein 10-7 to 10-5 M, and glyphosate 10-11 to 10-9; the concentrations were varied with a fixed ratio of both compounds. The results showed significant enhancement of ERE activation in the combination of 10-10 M glyphosate with 10-6M genistein and 10-9 M glyphosate with 10-5M genistein. At 10-7M genistein and 10-9M glyphosate, cell proliferation was increased to 169 % of control, where individually, the promotion was 145 %.
The important new finding is that glyphosate mimics oestrogen activity at minute concentrations; it may be inhibitory for oestrogen at high concentrations (while other toxicities (including oestrogen-independent carcinogenicity) also come into effect. Nonlinear concentration dependence is characteristic of environmental pollutants with endocrine disrupting effects (see [60]).
As stated in the IARC review [10], there is strong evidence that both glyphosate and glyphosate formulations cause genotoxicity. The end-points evaluated include biomarkers of DNA adducts and breakage, and various kinds of chromosome damage. Tests in bacteria gave consistently negative results.
The evidence base for glyphosate includes human cells in vitro, mammalian models in vivo and in vitro, and studies in non-mammals. In vivo studies in mammals generally gave positive results in the liver and mixed results in kidney and bone marrow.
There were three studies on residents in communities exposed to glyphosate-based formulations, two of which reported positive results. Additional evidence comes from studies that gave largely positive results in human cells in vitro, as well as in non-mammalian organisms.
For AMPA, the evidence for genotoxicity is moderate; while the number of relevant studies is not large, all gave positive results.
There is strong evidence that glyphosate, glyphosate-formulations and AMPA cause oxidative stress in human cells in vitro, and in non-human mammalian systems and non-mammalian organisms in vivo.
Studies on humans exposed to glyphosate contamination through aerial or ground spraying clearly show that glyphosate in the air is absorbed into the body and transported into cells.
A study [61] carried out in Ecuador on an exposed group of 24 randomly selected individuals living 3 km or less from an area on the border between Ecuador and Colombia where aerial spraying with a glyphosate formulation (Roundup Ultra) had occurred continuously for three days between December 2000 and March 2001, and sporadic aerial spraying continuing for three weeks following continuous spraying. A clinical history was completed for each of the exposed individuals and a wide-range of reactions were noted, including intestinal pain and vomiting, diarrhoea, fever, heart palpitations, headaches, dizziness, numbness, insomnia, sadness burning of eyes or skin, blurred vision, difficulty in breathing and blisters or rash. The unexposed control group consisted of 21 unrelated healthy individuals living 80 km away from the spraying area; they were similar to the exposed group regarding demographic characteristics and occupation but were not matched controls. Significant increase in DNA damage was found with the comet assay. The migration length of the exposed group was 35.5 + 6.4 μm, compared with the control of 25.94 + 0.6 μm. The results were highly significant at p<0.001.
A large study on community residents involved 137 women of reproductive age and their 137 spouses from five Colombian regions. In three regions with exposures to glyphosate formulations from aerial spraying, blood samples were taken from the same individuals at three time points (before spraying base line, 5 days and 4 months after spraying) to determine the frequency of micronucleus formation in lymphocytes. The baseline frequency of bi-nucleated cells with micronuclei was significantly higher in subjects from the three regions sprayed with glyphosate formulations and in a fourth region with pesticide exposure but not through aerial spraying, compared with a reference region without pesticides being used. The frequency of micronuclei in peripheral blood lymphocytes was significantly increased compared with baseline level in the same individuals after aerial spraying with glyphosate based formulations in each of the three regions (p= 0.01 to < 0.001) [62].
A study published in 2015 (not included in the IARC report) assesses damage to the genetic material of children exposed to pesticides in the province of Córdoba by determining the frequency of micronuclei in the cells lining the inside of the mouth [63]. The researchers found that children living within 500 m of spraying areas have over 66 % more cells with micronuclei than those living more than 3 000 m away. In addition, 40 % of the exposed children suffer from persistent conditions that may be associated with chronic pesticide exposure including respiratory symptoms, with and without additional symptoms such as skin itching or stains, nose itching or bleeding, lacrimation, eye and ear burning or itching. This study highlights the extensive area (500 km) affected by aerial spraying.
In studies on human cells, glyphosate induced DNA strand breaks (measured by comet assay) in liver Hep-2 cells, lymphocytes, GM38 fibroblasts, HT1080 fibrosarcoma cell line, and TR146 buccal carcinoma cell line. DNA strand breaks were also induced by AMPA in Hep-2 cells, and by a glyphosate-based formulation in TR146 buccal carcinoma cell line. In human lymphocytes, AMPA but not glyphosate induced chromosomal aberrations. Glyphosate did not induce a concentration-dependent increase in micronucleus formation in human lymphocytes at levels estimated to correspond to occupational and residential exposure. Sister chromatid exchange was induced by glyphosate and a glyphosate-based formulation in human lymphocytes (reviewed in [10, p. 46]).
In mammalian model systems in vivo conflicting results were obtained for the genotoxicity of glyphosate and glyphosate formulation (reviewed in [10, pp. 46, 48]).
In contrast, the evidence of genotoxicity in non-mammalian organisms is very extensive (reviewed in [10, pp. 48, 51]). For fish strand breaks in comet assay was consistently observed in several species, sabalo, European eel, zebrafish, Nile tilapia. AMPA also induced DNA strand breaks in the comet assay in European eel. A glyphosate-based formulation produced DNA stand breaks in numerous fish species including European eel, sabalo, guppy, bloch, neotropical fish Corydoras paleatus, carp, and goldfish. AMPA induced erythocytic nuclear abnormalities in European eel, micronucleus formation by different glyphosate based formulations in various fish.
Glyphosate-based formulations induced DNA strand breaks in caiman, frog, tadpoles and snail, but not in oyster, clam and the mussel larva. In earthworms one glyphosate formulation induced DNA strand breaks while two others did not, highlighting the potential importance of components other than the active ingredient.
Micronucleus formation was induced by a glyphosate formulation in earthworms and by a different glyphosate formulation in caiman and frog.
In the standard Drosophila melanogaster test, glyphosate induced mutation, but not in a cross of flies characterized by an increased capacity for CYP450-dependent bio-activation. A glyphosate formulation also caused sex-linked recessive lethal mutations in Drosophila.
In plants, glyphosate produced DNA damage in Tradescantia (spiderwort) in comet assay. Chromosomal aberration was induced after glyphosate exposure in fenugreek and in onion in one study but not in another. A glyphosate formulation induced chromosomal aberration in barley roots and onion but not in Crepis capillaris (hawksbeard). Micronucleus formation was not induced by glyphosate in Vicia faba bean or by a glyphosate formulation in Crepis capillaris.
The results from non-human mammalian cells in vitro generally gave positive results for genotoxicity (see [10, p.48]). In the most recent publication reviewed, IARC has not fully described the results which are quite important, as they address non-additive, and potential synergistic effects of mixtures of pesticides, as would be encountered frequently in the environment. Researchers at Aix-Marseille Université in France investigated the genotoxicity of mixtures of glyphosate and atrazine (the world’s top two herbicides) and their breakdown products AMPA and desethyl-atrazine (DEA) before and after photoactivation in hamster ovarian CHO K1 cells, in order to mimic real environmental conditions of exposure [64]. ROS (reactive oxygen species) were measured in the dark to assess oxidative stress, and micronucleus formation assayed for clastogenic (chromosomal abnormality) effect. They found that AMPA has a strong photo-inducible clastogenic effect, with MCC (minimal clastogenic concentration, the lowest concentration of pesticide that induced a significant increase of micronucleated cells) of 0.006 μg/mL in the dark, and 0.0004 μg/mL after light irradiation. Atrazine and glyphosate displayed cytogenetic activity only after metabolic activation, with MCC of 0.064 μg/mL and 5.8 μg/mL respectively. DEA was inactive in all experimental conditions. Surprisingly, combinations of two pesticides showed globally lower effects than those obtained with the most active individual compounds, AMPA and atrazine. Only atrazine+AMPA giving MCC of 0.39 μg/mL in the dark, and 0.0026 μg/mL after light stimulation and glyphosate+DEA giving MCC of 22.1 μg/mL after metabolic activation. In combinations of three pesticides, glyphosate+atrazine+AMPA gave a strong cytogenetic effect in the dark, with a MCC of 0.001 μg/mL; and all the combinations were activated by light. However, their cytogenetic potentials were close to AMPA, indicating weak synergistic effects. The mixture of 4 pesticides on the other hand exhibited a very powerful cytogenetic activity with MCC < 0.001 μg/mL under all experimental conditions. The MCC of 0.0004 μg/mL was 20-fold lower than that of AMPA in the dark, and at 0.0003 μg/mL, 200-fold lower than that of atrazine after metabolic activation. It was also strongly photo-stimulated, as the MCC was reduced by 100-fold by light to 4. 10-6 μg/mL.
The oxidative stress induced by the pesticides and pesticide mixtures measured in the dark showed that only AMPA gave an elevated oxidative effect, whereas the oxidative potencies of glyphosate, atrazine and DEA were very low. Among pesticide mixtures, atrazine+AMPA and glyphosate+atrazine+AMPA showed high oxidative potencies. But the mixture of all four exhibited the strongest oxidative potency of all. The results are summarized in Figure 3, where the Cytogenetic Potency (CP) is defined as the slope of the dose–response curves.
The results confirm that glyphosate, atrazine and AMPA have cytogenetic effects in mammalian cells; they show that mixtures of pesticides could have enhanced synergistic effects, and that sunlight could greatly amplify those effects. The results also show that the most genotoxic pesticide mixtures induce the most oxidative stress in the cells, suggesting that oxidative stress (see below) could play an important role in genotoxicity.
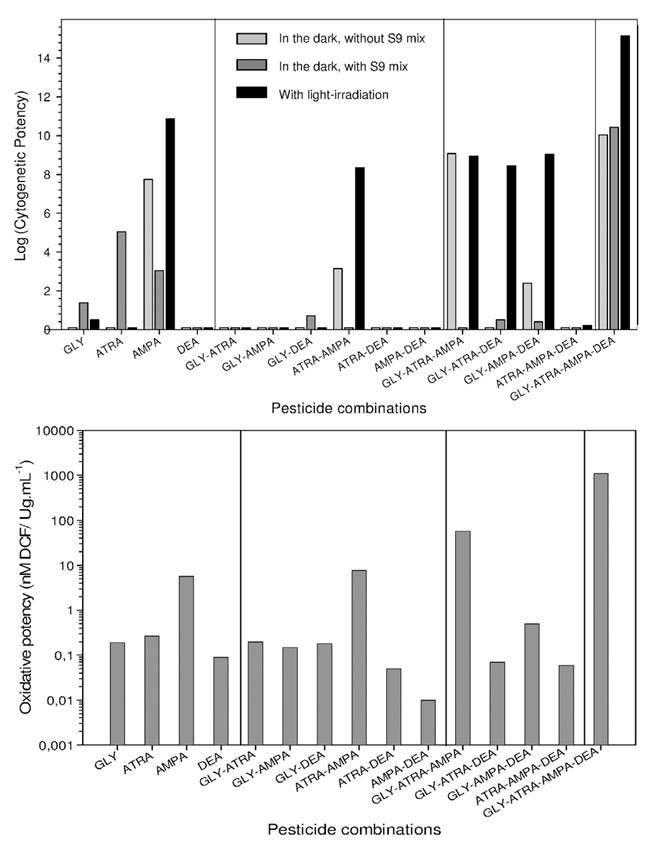
Figure 3 Cytogenetic potential (top) and oxidative potency (bottom) of pesticides and mixtures of pesticides
There has been no study on oxidative stress in humans as the result of exposure to glyphosate. Glyphosate and/or its formulations as well as AMPA produce oxidative stress in human cells. Human keratinocyte cell line HaCaT showed signs of oxidative stress in several studies with glyphosate or glyphosate formulation that was relieved or prevented by antioxidants (reviewed in [10, p. 68]).
In a study on human liver carcinoma HepG2 cells at the City University of Buenos Aires, Argentina, the glyphosate formulation Roundup UltraMax, Monsanto produced a 40 % increase in reactive oxygen species at concentrations well below that of agricultural use (40 mg/L), but neither glyphosate nor AMPA did even at concentrations of 900 mg/L [65]. Moreover, the glyphosate formulation induces dose-dependent cytotoxicity with an estimated LC50 value of 41.22 mg/L for 24 h exposure, predominantly through a caspase-dependent apoptotic pathway. This shows the importance of ‘inert’ adjuvants in contributing to the toxicity of glyphosate, or in being toxic themselves, which have been ignored in risk assessment of pesticides so far. In another publication from the same research team using Hep2 cell line (originating from human laryngeal carcinoma), the LC50 of Atanor (glyphosate formulation), Impacto (spray adjuvant) and the mixture of both [66]. The results showed that all of the three induced dose and time-dependent cytotoxicity and the toxicity of Atanor and Impacto was additive. All of them also triggered the apoptosis pathway. Furthermore all of them produced an increase in catalase and glutathione levels (markers of oxidative stress), with increase in ROS production in cells treated with Atanor and the mixture.
In primary lymphocyte cultures and plasma obtained from healthy male non-smoking blood donors, oxidative DNA damage in lymphocytes and lipid peroxidation in plasma were both significantly increased at glyphosate concentration of 580 mg/L (~3.4 mM), but not at lower concentrations [67]. In human erythrocytes isolated from healthy donors, production of reactive oxygen species was increased by glyphosate (> 0.25 mM), AMPA (> 0.25 mM), and N-methylglyphosate (> 0. 5 mM) [68].
Most studies of oxidative stress in mammals were conducted in rats and mice. It was found that glyphosate induced production of free radicals and oxidative stress in mouse and rat tissues through alteration of antioxidant enzyme activity, depletion of glutathione and increases in lipid peroxidation (reviewed in [10, p. 69]).
Positive associations between glyphosate and oxidative stress were reported in aquatic organisms; consistently presenting evidence that glyphosate can cause oxidative stress in fish. Similar effects were found in tadpoles and pacific oyster exposed to a pesticide mixture containing glyphosate (reviewed in [10, p. 70]).
The single study on mammalian cells [64] has been described in detail at the end the previous section.
The WHO IARC reclassified glyphosate as ‘probable carcinogen’ based on ‘limited evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals’, supported by strong evidence that glyphosate and glyphosate formulations are genotoxic, and strong evidence that glyphosate and its metabolite AMPA, and glyphosate formulations cause oxidative stress; both oxidative stress and genotoxicity being key characteristics of carcinogens.
Regarding carcinogenicity in humans, we have reviewed the main evidence presented in the glyphosate part of the IARC Monograph 112 [10], which showed that glyphosate exposure is associated with increased risk of non-Hodgkin’s lymphoma from several large epidemiological studies as well as smaller studies, and single studies have found non-significant RRs or ORs for glyphosate exposure and several cancer sites. In addition, we have presented further relevant evidence from the formal scientific literature as well as reports from non-government organizations.
First, glyphosate use has gone up rapidly and enormously worldwide especially since glyphosate-tolerant genetically modified crops were introduced. The global glyphosate market demand in 2012 was 718 600 tonnes [35], with GM crops accounting for 45.2 % of the total demand, and glyphosate for ~25 % of the global pesticide market [36]. Glyphosate and its residues have heavily contaminated air, soil, and water worldwide, constituting a major increase in pesticide burden on public health. It is to be found not only generally in human and livestock urine through exposure in food and feed (as well as in drinking water, and through inhalation from the air and absorption through the skin), but also in all livestock tissues tested and in mother’s milk, contradicting all the claims of the manufacturer that glyphosate does not accumulate in soil or leach into water, and that it does not bio-accumulate in tissues.
Second, although no post-market health monitoring has been done for either GM crops or glyphosate, it is significant that US government data show a marked deterioration of public health, with increase in incidence of 22 diseases including 6 cancers – liver, thyroid, bladder, pancreas, kidney and myeloid leukaemia - closely tracking the increase in GM crops planted and glyphosate used in the country [8]. For 22 diseases, the Pearson correlation coefficients were calculated between incidence and % GM crops and between incidence and glyphosate usage. Most of the 44 coefficients are greater than 0.91, with none of them falling below 0.81, and those for incidence of cancers and glyphosate use among the highest.
Similarly, In Argentina, where the use of pesticides including especially glyphosate herbicides has increased more than 8.5-fold since GM crops were introduced 20 years ago (Chapter 3 of [9]), physicians and local governments have been documenting rapid increases in birth defects and cancers for years. An official report from the province of Chaco recorded a 4.5-fold increase in the incidence of birth defects over 12 years, from 19.1 /10 000 in 1997 to 28.1 /10 000 in 2001 and 85.3/ 10 000 in 2009 [43]. Also, the incidence of childhood cancer almost doubled from 8.03/ 100 000 in 1991 to 11.2/100 000 in 2001 and 15.7/100 000 in 2007. A second report released by the Ministry of Health in Córdoba shows the highest rates of deaths from tumours occur in areas where GM crops and agro-chemicals are used, and they are almost double the national average [44].
Finally, a study in an animal model that includes proteomic analysis suggests that a glyphosate formulation can promote cancer in a similar way to a known cancer promoter [51], while studies in human cells show that glyphosate at minute concentrations can promote the growth of oestrogen dependent breast cancer cells [59], and at much higher concentrations promotes growth of cancer cells by a hormone independent mechanism [58].
With regard to animal experiments, we have reviewed the long-term feeding studies assessed by the IARC that showed positive results for cancers. These include an experiment submitted to the EPA on male and female mice showing a significant increase in carcinoma of the renal tubule as well as significant increase in combined carcinoma and adenoma of the renal tubule in male mice. A second experiment on mice submitted to Joint FAO/WHO Meeting on Pesticide Residues (JMPR) found a significant increase in the incidence of haemangiosarcoma as well as a non-significant increase in the incidence of histiocytic sarcoma in the lymphoreticular/haemopoietic tissues in males. An experiment on rats submitted to the EPA found a significant increase in the incidence of pancreatic islet cell adenoma compared with controls in males at both the lowest and highest doses. There was also a statistically significant positive trend in the incidence of hepatocellular adenoma in males and of thyroid follicular cell adenoma in females. A second experiment on rats submitted to the EPA also found an increase in the incidence of pancreatic islet cell adenoma in all dosed males, which was statistically significant at the lowest dose.
The experiment on cancer promotion in mice skin [51] has been mentioned above.
In addition, we have drawn attention to a review on glyphosate toxicity published in 1995 [52], which showed how the EPA dismissed successive animal studies (including one that was not assessed by IARC showing testicular tumours in dosed male animals), finally resulting in altering the original classification of glyphosate as ‘possible carcinogen’ to ‘noncarcinogenic’ in 1993, against the advice of its own scientists, as documented in memos from the EPA archives. This misclassification has been largely responsible for two decades of people and planet being subjected to chronic glyphosate exposures in allowing the manufacturer to claim glyphosate ‘safe’, and perpetrating many other falsehoods in promoting its ubiquitous and liberal use.
There is copious evidence on the genotoxicity of glyphosate and glyphosate formulations in human cells in vivo and in vitro, and non-mammalian organisms in vivo. There have been no studies on oxidative stress in human cells in vivo as the result of exposure to glyphosate. Many studies showed that glyphosate and/or its formulations as well as AMPA produce oxidative stress in human cells, in mammalian models, as well as various species of fish and other aquatic organisms.
We have added a study published in 2015 (not included in the IARC report), which found that children living within 500 m of spraying areas have over 66 % more cells with micronuclei in in the cells lining the inside of the mouth than those living more than 3 000 m away [63], and 40 % of the exposed children suffer from persistent conditions that may be associated with chronic pesticide exposure. This study highlights the extensive area (500 km) affected by aerial spraying.
Further, we have elaborated on a published study dealing with an aspect ignored in the IARC report, i.e., synergistic effects of mixtures of herbicides most likely to be encountered in the environment. The study investigated the genotoxicity of mixtures of glyphosate and atrazine (the world’s top two herbicides) and their breakdown products AMPA and desethyl-atrazine (DEA) before and after photoactivation in hamster ovarian CHO K1 cells [64]. It found that the mixture of 4 pesticides exhibited a very powerful genotoxic activity 20 times that of AMPA (the most genotoxic agent) and 200-fold that of atrazine after metabolic activation, and which was further enhanced 100-fold by light. The genotoxicity of the herbicides and mixtures was accompanied by corresponding level of oxidative stress induced.
We suggest that the additional evidence – had it been taken into proper account – would have been sufficient to classify glyphosate as definitely carcinogenic.
It should be noted that chronic exposure to glyphosate herbicides is associated not only with cancers, but also with infertility, impotence, abortions, birth defects, neurotoxicity, hormonal disruption, immune reactions, an unnamed fatal kidney disease, chronic diarrhoea, autism and other ailments. In addition to human diseases, glyphosate herbicides are linked to more than 40 new and re-emerging major crop diseases. They are causing irreparable harm to the entire food web; including the plant kingdom, beneficial microbes that supply nutrients to our crops and soils, fish and other aquatic life, amphibians, butterflies, bees, birds, mammals, and the human microbiome (reviewed in Chapter 1 of [9]). Indeed, there is a strong case for a worldwide ban on glyphosate.
Total bans on glyphosate are already in place or announced in El Salvador, Bermuda, and Sri Lanka, and proposed in other countries; while a number of partial bans have also been imposed including a ban on aerial spraying in Columbia (see [69] Fallout from WHO Classification of Glyphosate as Probable Carcinogen, SiS 67). The Californian EPA announces it plans to label glyphosate “known to cause cancer” [70]. We need to stop the devastation of people and planet that has gone on for the past 20 years by halting the use of glyphosate and shifting comprehensively to sustainable, organic non-GM agriculture [71] (Food futures now, organic, sustainable and fossil fuel free, ISIS/TWN special report) already shown to be the most effective way to feed the world with healthy uncontaminated food, and to mitigate and adapt to climate change.
Article first published 09/09/15
Comments are now closed for this article
There are 1 comments on this article.
Lia Giraldo da Silva Augusto Comment left 12th September 2015 03:03:28
Yes, in our laboratory we did a good revision and we agree with IARC classification