New Age of water
Entire biochemistry and cell biology textbooks will have to be rewritten on how water in the cell and extracellular matrix is stage-managing the drama of life. This continues the exclusive series started in SiS 23.
Prof. Martin Chaplin presents a new theory on the structure of water in the cell that switches between low-density and high-density clusters
Although we understand much of what goes on inside cells at a molecular level, we don't know how all the molecules can work together as a whole. Much useful biochemistry has been discovered using dilute preparations from homogenised dead cells, but living cells are very different, and contain more concentrated solutes and more organised proteins. Indeed, test tube experiments may mislead us, and it should come as no surprise to find that living cells possess characteristics that are very much more than the sum of their parts.
The study of the live cell is fraught with difficulty, as most procedures change it from its native state. The key to understanding the cell comes from acknowledging the one constituent that has often been ignored: water. The significance of water for the cell becomes clear when we seek to solve big puzzles, such as 'How are potassium ions able to maintain a high concentration inside cells whereas sodium ions are found mainly outside?' and 'How do cells remain functional even when large holes are made in their surface membranes?'
There are at least four views as to how the water inside the cell affects its function:
The theory that I shall describe in this article (which I presented at the Gordon Research Conference on Interfacial Water and Cell Biology in June 2004) belongs to the fourth, new category. I propose that changes in the density and clustering of intracellular water are modulated by the mobility of key proteins, which in turn are controlled by the energy status and ionic content of the cell.
Water possesses many properties that seem strange, or anomalous [3]. Some of these, such as its high melting and boiling points can be simply explained as due to water's hydrogen bonded clustering. Over the last 10 years, a broad range of evidence has accumulated concerning a two-state structuring within liquid water, which can explain many of the remaining anomalies [4, 5]. This theory involves the presence in liquid water, of clusters with a lower density comparable with that of ice. The water molecules in such clusters flicker between partners as their hydrogen bonds are constantly making and breaking. Over a long time scale, they appear as favoured arrangements. These low-density water clusters do not consist of ice-like crystals, due to their lack of long-range order, but they do contain water molecules linked by hydrogen bonds in an expanded, 4-coordinated tetrahedral arrangement. At the smallest scale, the water may be thought of as an equilibrium between two water tetramers (see Fig. 1): structure A, held closely by non-bonded interactions, forming a more dense structure, and structure B, with molecules held further away and linked by hydrogen bonds to form a less dense structure There is little difference in energy between the structures A and B, so the equilibrium is easily affected by the presence of solutes and surfaces. An increase in temperature or pressure will shift the equilibrium to the left.
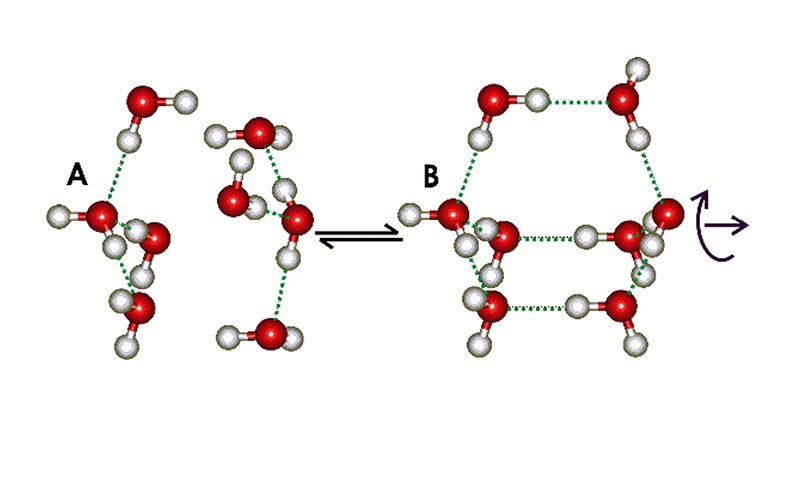
Figure 1. Equilibrium between two water tetramers.
Although the natural structuring in water at ordinary temperatures tends towards the 'collapsed' structure A, the low density structure B can grow to form larger non-crystalline clusters based on dodecahedral (12-sided) water cluster cores, similar to those found in the crystalline 'clathrate hydrates'; as for example, the extensive icosahedral (H2O)280 aggregate built up from tetrahedrally hydrogen-bonded water molecules surrounding a dodecahedron made up of 20 water molecules, the basic clathrate cage (Fig. 2).

Figure 2. Extensive icosahedral (H2O)280 structure of water built up from tetrahedrally hydrogen-bonded water molecules.
The differences in intracellular and extracellular environments of cells is primarily due to the extensive surface area and high intracellular concentration of solutes that promote the low-density clustering of water and restricted diffusion inside cells. The extensive surface of cellular membranes (e.g., each liver cell contain ~100 000 mm2 membrane surface area) favours the formation of low-density water inside cells, as the membrane lipids contain hydrophilic head groups that encourage this organization of the associated interfacial water. Other surfaces attract the water, so stretching the hydrogen-bonded water contained by the confined spaces within the cells.
The difference in ionic concentrations is particularly evident in sodium (Na+; intracellular, 10 mM; extracellular, 150 mM) and potassium (K+; intracellular, 159 mM; extracellular, 4 mM). Na+ ions create more broken hydrogen bonding and prefer a high aqueous density, whereas K+ ions prefer a low-density aqueous environment, as proven by Philippa Wiggins [6]. The differences in intracellular and extracellular distributions of potassium and sodium are due to differences in the affinity of these ions for water. The interactions between water and Na+ are stronger than those between water molecules, which are in turn stronger than those between water and K+ ions, all due to the differences in surface charge density of the ions - that of the smaller Na+ ion being nearly twice that of K+ ions. Ca2+, with an intracellular concentration 0.1 mM and an extracellular concentration of 2.5 mM, has a surface charge density more than twice that of Na+, and has even stronger destructive effects on low-density hydrogen-bonding than Na+ ions.
Other studies confirm the preference of K+ ions for low-density water over Na+ ions. The ions partition according to their preferred aqueous environment; in particular, the K+ ions are preferred within the intracellular environment and naturally accumulate inside the cells at the expense of Na+ ions. This process occurs simply as a result of the water structuring without the help of putative ion-pumps in the cell membrane.
Besides, membrane ion-pumps cannot produce these large differences in ionic composition, simply because the (ATP) energy required far exceeds the energy available to the cell. Also, many studies, as for example, the extensive series carried out by Gilbert Ling, have shown that cells do not need an intact membrane or active energy (ATP) production to maintain the ionic concentration gradients.
The degree to which the density of cell water is lowered is determined by the solutes and the state of motion of protein. Water has conflicting effects in the mixed environments around proteins due to the variety of amino acids making up their surfaces. Weak interactions between the protein and surface water molecules allow greater protein flexibility. Strong interactions endow the protein with greater stability and solubility.
There is generally an ordered structure in the layer of water molecules immediately surrounding the protein, with both hydrophobic clathrate-like and hydrogen bonded water molecules each helping the other to optimize water's hydrogen bonding network. Protein carboxylate groups are generally surrounded by strongly hydrogen-bonded water whereas the water surrounding the basic groups arginine, histidine and lysine tends towards a more-open clathrate structuring. The formation of partial clathrate cages over hydrophobic areas maximizes non-bonded interactions between the water and the protein without loss of hydrogen bonds between the water molecules whereas carboxylate groups usually only fit a collapsed water structure (see below) creating a reactive fluid zone.
The rotation of the proteins will cause changes in the water structuring outside this closest hydration shell. At the breaking surface, hydrogen bonds are ruptured, creating a zone of higher density water. Protein rotation thus creates a surrounding high-density water zone with many broken hydrogen bonds.
Protein has two acidic amino acids, aspartate and glutamate, with carboxylate (-CO2-) side chains. Normally, aqueous hydrogen bonding to these carboxylate oxygen atoms both attracts water molecules causing a localised high density water clustering and reduces the acidity of the carboxylic acids. Otherwise, when the surrounding water molecules prefer to hydrogen bond to themselves as with the formation of a clathrate cage, the acidity of the carboxylate groups is increased. It is found that Na+ ions prefer binding to the weaker carboxylic acids whereas K+ ions prefer the stronger acids [1].
Na+ and K+ ions also behave differently when close to the carboxylate groups; K+ ions have a preference for forming ion pairs, where there is direct contact between the K+ and carboxylate ions, whereas Na+ ions form solvent separated pairings where water molecules lie between the Na+ and carboxylate ions, forming strengthened hydrogen bonds to the carboxylate groups [7]. This is due to the Na+ ions holding on to their water more strongly. The K+ ions prefer to be within a clathrate water cage and this preference both reinforces its direct ion pairing to the carboxylate group and discourages aqueous hydrogen bonding to the associated carboxylate groups.
The direct association of K+ ions with the aspartate and glutamate groups in proteins is the central theme of Ling's fixed charge hypothesis where evidence for the molecular mechanism for the association includes (1) the low intracellular electrical conductance, (2) the strongly reduced mobility of intracellular K+ ions, (3) the one to one stoichiometric absorption of K+ ions to the carboxylate groups and (4) identification of the K+ ion absorption sites as the aspartate and glutamate side chains of the intracellular proteins.
Actin is a highly conserved and widespread eukaryotic protein (42-43 kDa) responsible for many functions in cells. Non-muscle cells contain actin in amounts 5-10% of all protein, whereas muscle cells contain about 20%. Actin is converted between a freely rotating monomer molecule (G-actin; about 4 - 6 nm diameter) and a static right-handed double helical polymer protein filament (F-actin; up to several microns in length) by ATP; a process involving the conversion of an a-helix to a b-turn in one of its structural domains. Each molecule of the freely rotating G-actin can stir a large volume of water, whereas F-actin has a much more ordered structure so creating more order in its surrounding water. The protein fibres trap water, reducing its movement and compensated by greater hydrogen bonding. Also, capillary action stretches the confined water, so ensuring that it is of lower density and hence more highly structured than the bulk water.
All actin molecules contain a conserved negatively charged N-terminus, for example the N-acetyl-aspartyl-glutamyl-aspartyl-glutamyl sequence in rabbit muscle a-actin. When G-actin polymerises in the cell under the action of ATP to form F-actin, this highly carboxylated antenna is placed on the exposed outer edge of the helix, where it may be additionally used as a binding site for other proteins, such as myosin. Tubulin, another intracellular structural protein that forms immobile structures within cells, possesses an even more extensive negatively charged acidic C-terminal conserved antenna of about eight carboxylate groups that serves similar functions.
F-actin's multiply negatively charged N-terminus attracts positively charged cations. Under conditions when the carboxylic acids are weaker, both K+ and Na+ ions may form solvent separated species. This competition results in a preference for Na+ ions and high-density water. However, the natural rotation of the protein will tend to sweep such ions, and their associated water, away. If the protein is prevented from rotating, Na+ ions tend to destroy any low density structuring around carboxylate groups of the protein. However, the intracellular Na+ ion concentration is generally far lower than that of K+ ions, which allows K+ ions to compete successfully for these sites, forming ion pairs and encouraging clathrate formation.
Binding of K+ ions by the carboxylate groups lowers the ionic strength of the intracellular solution. As this ionic strength decreases, the acidity of phosphate groups decreases, resulting in the conversion of the intracellular doubly charged HPO42- ions to the singly charged H2PO4- ions, more favourable to low density water clustering. All intracellular phosphate entities will behave similarly. The cooperative effects of the change between static filament formation and freely diffusional protein are summarized in Fig. 3.
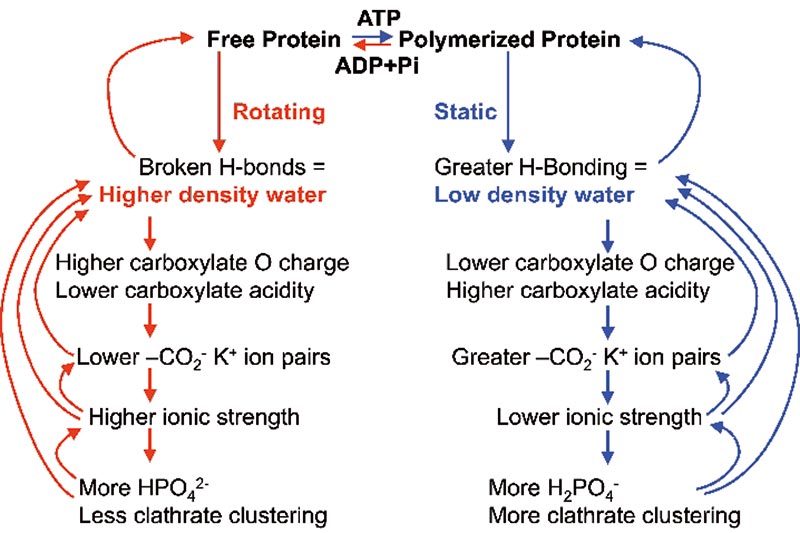
Figure 3. A summary of the cooperative effects when mobile proteins such as actin are polymerised,
Formation of K+-carboxylate ion pairs leads to the formation of a surrounding clathrate water structuring that further guides icosahedral water structuring (so ensuring maximal hydrogen-bond formation) and informing neighbouring carboxylate groups. This signalling cooperatively reinforces the tetrahedrality of the water structuring found between these groups. The clathrate cage allows rotational mobility (like a ball-and-socket joint), enabling the hydrogen bonding to search out cooperative partners (Fig. 4).
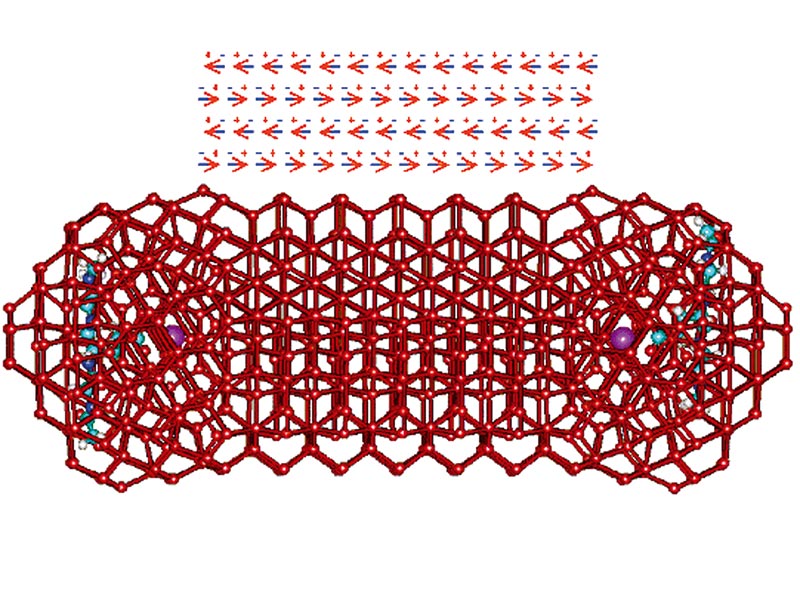
Figure 4. This diagram shows the clustering around two K+-carboxylate ion pairs (about 4 nm apart) as may be attached to part of two protein's structures. There are 7-8 shells of water around each surface as is typically found between intracellular proteins. The K+ ions are shown as violet and the water network is shown as linked (i.e. hydrogen bonded) oxygen atoms (shown red) without showing their associated hydrogen atoms. The hydrogen bonding initially forms clathrate cages around the ion pairs, followed by a more extensive icosahedral arrangement. This is then followed by extension of the hydrogen bonding along 'rays' connecting the neighbouring sites. Once these 'rays' link, the hydrogen bonding of each reinforces the other in a cooperative manner, so strengthening the linkage and reinforcing the overall low density aqueous environment. As the aqueous clathrate cage possesses a more negative charge on its interior and a more positive charge on the outside, there is a marked polarization in the water molecules that reinforces the hydrogen bonding interactions.
Although the clustering involves a major drop in aqueous mobility, the stronger 4-coordinated bonding compensates this. This theory offers a molecular explanation for Ling's association-induction polarized multilayer model (see "Strong medicine needed in cell biology", this issue). The initial icosahedral size (3 nm diameter), surrounding each ion pair, also equals the water domain size proposed by John Watterson. The tetrahedral structuring possesses five-fold symmetry, which prevents easy freezing in line with the pronounced supercooling found for intracellular water.
Extension of the clathrate network and its associated low density water enables K+ ion binding to all aspartic and glutamic acid groups, not just the key ones within the crucial N-terminal acidic centres. Thus, the sol-gel transition of Pollack (see "Biology of least action", SiS 18) may be interpreted as due to the formation of low density water clustering (the gel state) due to clathrate clustering around K+-carboxylate ion pairs.
In the presence of raised levels of Na+ and/or Ca2+ ions, as occasionally occurs during some cell functions, these ions will replace some of the bound K+ ions. These newly formed solvent separated Na+ and/or Ca2+ ion pairings destroy the low-density clathrate structures and initiate a cooperative conversion of the associated water towards a denser structuring.
In conclusion, the aqueous information transfer within the cell involves the following:
Article first published 13/10/04
Martin Chaplin is Professor of Applied Science, London South Bank University, UK, with special interests in the interactions between water and biological molecules.
Comments are now closed for this article