I-SIS Exclusive!
Just add sunlight for energy and life Dr. Mae-Wan Ho
Why does water vaporized into the sky form clouds instead of just spreading out evenly in space? Where does lightning come from in a storm? How does Jello hold so much water inside without it leaking out? How does the water bug walk on water?
If you never had your curiosity aroused by these natural phenomena that have exercised generations of scientists still in search of a definitive answer, try this.
Two identical beakers are almost filled with water and placed next to each other with the rims touching. The beakers of water are connected to a power pack and a current is passed through a positive electrode placed in one beaker and the negative electrode in the other. Instantly, a bridge of water forms between the beakers, looping over the adjoining rims and connecting the two bodies of water. The beakers are then moved apart slowly, the water bridge stretches and lengthens, but remains intact, even when the beakers are separated by a gap of several centimetres. And furthermore, the water bridge is still passing electricity from one beaker to the other, like a stiff, transparent cable. There is no doubt that water conducts electricity, as our readers will be aware [1] (Positive Electricity Zaps Through Water Chains, SiS 28). But what makes the water stiffen up to make a bridge?
The beginning of an answer to all of these questions, and the key to many more surprisingly phenomena readily demonstrated on the ordinary lab bench and some even on the kitchen table, turns out to be “liquid crystalline water”, water that is ordered and aligned like liquid crystals [2]. It gets my vote for the most significant discovery of the present century so far. It also turns out that liquid crystalline water and sunlight are practically all we need for energy and life.
Water is one of the simplest chemical compounds (see Fig. 1). Yet its remarkable ‘anomalous’ properties have resisted all attempts at a consistent scientific explanation; that is, until quite recently. A remarkable collection of dedicated researchers on ‘interfacial water’ have been homing in on the secret of water [3] (see New Age of Water series (SiS 23, 24, 28, 32); and one of them may have just got it.
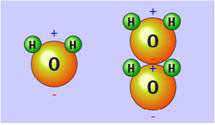
Figure 1. The water molecule with positive and negative charges at opposite ends and how it could stack up with opposite charges next to each other (courtesy of physicalgeography.net)
Gerald Pollack, Prof. of bioengineering, recently received the highest honour that the University of Washington at Seattle in the United States could confer on its own staff. He was to give the 2008 Annual Faculty Lecture on his research, entitled, “Water, energy and life: Fresh views from the water’s edge”. I watched the hour-long lecture via the video link [2] with great fascination.
I am no stranger to Pollack’s work, having reviewed his book, Cells, Gels and the Engines of Life [4] published in 2001 (see Biology of Least Action, SiS 18)[5]; and featured the amazing discovery from his laboratory a couple of years later [6] (Water Forms Massive Exclusion Zones, SiS 23).
What strikes me above all is the elegant simplicity of his experimental approach that takes our understanding of the most abundant, most vital substance for life on earth a quantum leap forward. Many of the experiments can be done on the kitchen table, and you don’t even need a microscope to see the results. Add to that a highly congenial and unassuming personality, and no wonder Pollack is attracting undergraduates and graduates like flies, not to mention many collaborators around the world.

The initial discovery that Pollack and his colleague Zheng Jian-ming reported in 2003 [6] was that water forms a massive ‘exclusion zone’ (EZ) next to the surface of hydrophilic (water-loving) gels. The EZ is so-called because it excludes solutes, i.e., substances dissolved in the water. By putting into the water solutes large enough to be seen under the microscope, or even with the naked eye, the EZ shows up as a region completely clear of the solute. Thus, when a suspension of microspheres 0.5 to 2 mm in diameter is put into a chamber with the gel, a clear zone, free of microspheres soon develops next to the gel and typically ends up hundreds of microns thick (see Fig. 2). This EZ is stable if undisturbed, for days and weeks once it is formed.
The scientific community greeted the initial discovery with much scepticism. Interfacial water – water next to surfaces – is generally recognized as being restricted in motion, relatively ordered, and having somewhat different properties from water existing in the bulk. Using sophisticated techniques and big machines such as NMR (nuclear magnetic resonance) X-rays, and more recently, neutron diffraction, researchers have found no more than one or two layers that have altered properties compared to bulk water [6]. But the EZ is so enormous that at least hundreds of thousands of layers are involved.
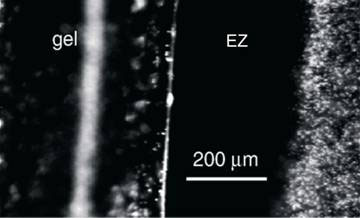
Figure 2. Clear exclusion zone next to gel surface free of microspheres
Gilbert Ling, doyen of the breakaway biological water researchers, had long argued that all water in the cell (typically 70 percent by weight) is ordered with very unusual properties [7] (see Strong Medicine for Cell Biology [8], SiS 24). More recently, Ling proposed on theoretical grounds that the ordered layers could extend infinitely under ideal conditions [9].
Pollack and his team spent a year ruling out all kinds of artefacts and extended their results, showing that the EZ of water is a very general phenomenon. What’s more, it had been discovered as far back as a hundred years ago; only to be consigned to oblivion after the ‘polywater’ controversy of the late 1960s, when the claim of ‘polymerised’ water was finally attributed to contaminants [10].
Pollack’s team found that a wide range of hydrophilic gels gave EZ in water: polyvinyl alcohol, polyacrylamide, polyacrylc acid, Nafion (used as a proton exchange membrane in fuel cells), and biological tissues such as a bundle of rabbit muscle or collagen [11]. In fact, a single layer of hydrophilic charged groups coated on any surface is sufficient to give an exclusion zone. The requirement is to have chemical groups that can form hydrogen bonds with water molecules. Similarly, solutes need not be microspheres, they could be red blood cells, bacteria [2], colloidal gold, and even molecules such as serum albumin labelled with a fluorescent dye, and a fluorescent dye molecule as small as 200-300 daltons. All of these are excluded from EZ water.
Most interestingly, EZ water was found at the air-water interface. The EZ layer, thick enough to be seen easily with the naked eye, was sufficiently stiff to be lifted up with a glass rod without breaking (Fig. 3). This readily explains how the strong surface tension of the EZ layer allows water bugs to walk over it without falling in. Also if such water forms next to hydrophilic surfaces inside the Jello, it would not fall out. And, we can see how the water bridge of EZ water could form between the separated beakers. Of course, an electric field will improve the alignment of the water molecules and hence its crystallinity and stiffness .

Figure 3. Glass rod lifts up stiff EZ layer at water air interface
Now that EZ water can be produced in bulk, it is easy to demonstrate other altered properties. NMR measurements confirm that the layer is associated with decreased mobility (increased ordering) relative to the bulk water, while infrared imaging showed it emitted much less than bulk water, again indicative of increased order.
Pollack refers to EZ water as “liquid crystalline water”, and says it was in fact biologist William Bate Hardy who first suggested almost a hundred years ago that water molecules at the interface could exist in many layers approaching crystalline order. This is very much in line with the discovery in my laboratory that organisms and cells are liquid crystalline [12] (The Rainbow And The Worm), and that water is intrinsic to the liquid crystallinity of organisms [13] (The Liquid Crystalline Organism and Biological Water, ISIS scientific publication).
But more surprises are in store.
There was already a hint that the EZ has unusual electrical potential when pH sensitive dyes were used as solutes to see if they too, were excluded from the EZ. Indeed, they were, but they also showed up a zone of unusually low pH (red band) right above the clear EZ (see Fig. 4). A low pH means high concentration of protons (H+) immediately next to the EZ, and decreasing away from it.
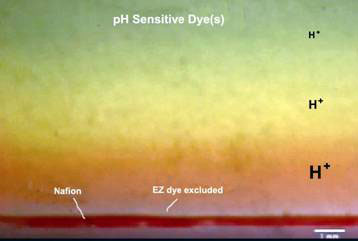
Figure 3. Proton rich region above EZ with dye excluded when a pH sensitive dye was used (still captured from video [2])
An excess of protons suggests that charge separation has taken place in the water molecules as follows:
H2O —› H+ + OH- (1)
So where did the negatively charged OH- ions go? A measurement of electrical potential shows that away from the EZ, the bulk solution had the same electrical potential everywhere, however, as soon as the measuring electrode enters the EZ, the electrical potential dropped sharply to –120mV or more, depending on the gel involved, remaining at that level well into the gel itself (see Figure 5).
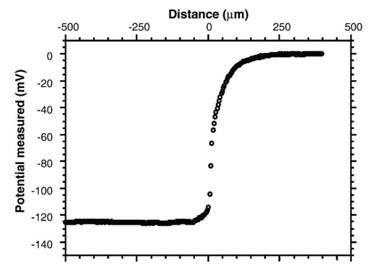
Figure 5 Electrical potential measured at different distances from the gel surface located at 0
This macroscopic separation of charges is stable, as is the EZ itself. It is in fact a water battery. A battery, like any other, could be used to power light bulbs or your labtop, and could be the most exciting application of liquid crystalline water (see Fig. 6). But what charges up the water battery? It takes energy to separate the charges, so where does the energy come from? That too was a surprise.
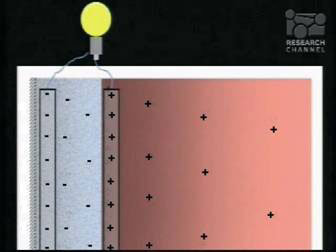
Figure 6. A water battery
It turns out that water is sensitive to light, as is revealed by the exclusion zone next to a gel. It thickens on being exposed to light, which means that light enhances the formation of liquid crystalline water. The entire spectrum of sunlight is effective, but the peaks are in the visible blue and especially the invisible near-infrared (3 000 nm) regions. A mere 5 minutes exposure to the infrared light will cause the EZ to thicken several-fold. And if you connect up the EZ and the bulk water above to an external circuit, there is a measurable current, which lasts for a considerable time after the infrared light is turned off.
Green plants and especially blue-green bacteria have been splitting water according to equation (1) for billions of years, in order to obtain energy from the sun; and in the process fixing carbon dioxide to make carbohydrates and other macromolecules to feed practically the entire biosphere. The separation of charges in the formation of liquid crystalline water is essentially the same process.
Pollack asks tantalisingly: Can water replace oil? The applications of liquid crystalline water are wide-open. His laboratory is already working on a water-purification device based on separating liquid crystalline water of the EZ from the bulk water. (Liquid crystalline water is reputed to have health-promoting properties, though that is still unconfirmed.) Another application is anti-fouling agent: a coating that essentially prevents any impurities in water from sticking.
One invention I would love is a web suit that would enable me to glide over the water like a water bug!
Pollack’s findings have fundamental implications for our understanding of physics, chemistry and biology.
One puzzle that is immediately solved is the formation of colloid crystals (see Fig. 7) – literally crystals made of colloid particles arranged in an orderly way in solvents - which is very topical in the manufacture of nano-structured electronic and photonic devices.
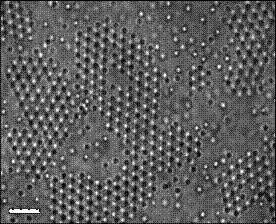
Figure 7. Colloid crystals, scale bar 20 microns
Norio Ise and his colleagues in Osaka, Japan, first discovered colloid crystals forming in water more than 20 years ago [14] (Water and Colloid Crystals, SiS 32), and they explained the colloid crystals in terms of a long-range attraction between the colloid particles, though the precise mechanism has remained elusive. The major difficulty is that the colloid particles have the same charge and it is impossible, according to conventional theory for like charges to attract one another.
Pollack’s findings provide just the mechanism required. Colloid particles and microspheres are like the hydrophilic gel surfaces that form layers of liquid crystalline water or EZ. In the case of the gel, the EZ has an excess of negative charges with excess positive charges in the region outside (see Fig. 6). In the case of the microspheres and colloid particles, each is enclosed in a shell of liquid crystalline water with excess negative charges, while the positive charges are also driven outside (see Fig. 8). The repulsion between the negatively charged particles is exactly balanced by the attraction to the positive charges in between. In the space between two particles, there will be an excess of positive charges compared to elsewhere, which is why the particles end up being attracted to one another.
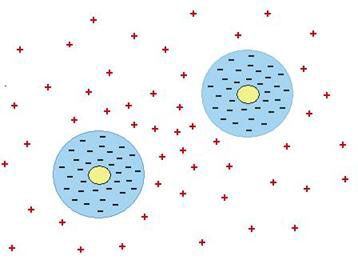
Figure 8. How like attracts like (see main text)
The same mechanism may explain why clouds form. Clouds are essentially minute water droplets nucleated on particles, and these too would end up attracting one another. The mechanism of charge separation explains at least where the enormous amount of energy unleashed in a lightning flash comes from. Storms could perturb the equilibrium of charged swarms in the atmosphere, leading to violent electrostatic discharges. The discharge heats up the air so much that it set up a shock wave, which is why thunder follows lightning. Obviously the details need to be worked out, but at least the major mechanism is clear.
The long-range attraction between like particles is also the main mechanism for self-assembly of molecules and particles inside the cells. It is the organizing principle that has long eluded biology, or as Albert Szent-Gyorgyi, Nobel Laureate and father of biochemistry said: “Life is water dancing to the tune of molecules.”
Perhaps it is the other way around as well: Life is molecules dancing to the tune of sunlight and water
Article first published 31/03/08
Comments are now closed for this article