Physics of Organisms
Quantum coherent delocalized protons make confined water nanotubes superconducting with large implications for cell biology Dr. Mae-Wan Ho
Some time ago, I suggested that water confined in carbon nanotubes could be superconducting ([1] First Sighting of Structured Water, SiS 28) based on proton jump-conduction along hydrogen-bonded chains of water molecules ([2] Positive Electricity Zaps Through Water Chains, SiS 28). And by analogy, the nanotubes of water interwoven with the triple-helix molecules of collagen molecules in the collagen fibres of extracellular matrix of all multicellular organisms may also be superconducting [3] (Collagen Water Structure Revealed, SiS 32). Within the past 10 years, there has been increasing recognition that liquid water may be quantum coherent even at ordinary temperatures and pressures ([4] Cooperative and Coherent Water, SiS 48).
Fresh evidence has now emerged that water confined in nanospace is both quantum coherent and proton superconducting by quantum delocalisation, which goes beyond classical jump conduction. This is a major boost for the new cell biology introduced in my new book [5] 'Living Rainbow H2O' (ISIS publication), in which water occupies centre stage (see [6] Living H2O, SiS 55).
The key to water’s remarkable properties is the hydrogen-bond interconnecting water molecules. It is usually regarded as classical and electrostatic; but many observations are inconsistent with that picture.
American chemist/biochemist and Nobel laureate Linus Pauling (1901-1994) was the first to suggest in 1935 that the hydrogen bond and covalent bond in ice may switch places in view of residual entropy (randomness) existing even at very low temperatures [7]. For example, the puckered six-member ring of water molecules in ice may switch from one configuration to the other by the protons covalently bonding to one or the other neighbouring oxygen (see Figure 1). Thus, the hydrogen bond is partly covalent.
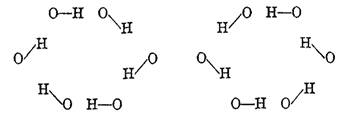
Figure 1 Protons in alternative bonding relationship to neighbouring oxygen in a 6-member ring of water molecules (from [7])
In 1999, researchers at Bell Laboratories New Jersey in the US teamed up with those at the European Synchrotron Radiation Facility of Grenoble in France, and the National Research Council of Canada in Ottawa, to study the hydrogen bond in ordinary ice Ih with inelastic X-ray scattering at the Grenoble facility [8]. In this technique, beams of X-rays are bounced off electrons with both the energy of the electron and the x-ray changed. They investigated the Compton profile - scattering intensity as a function of energy or momentum (mass x velocity) – at different orientations of a carefully prepared slab of ice, and noted the anisotropies (directional differences). They found periodic intensity variations in the anisotropies of the Compton profile peaking at distances of 1.72 and 2.85 Å, which are close to the hydrogen bond length and the nearest neighbour O-O distance respectively. This result was interpreted as direct evidence for the substantial covalent nature of the hydrogen bond. In support of their interpretation, they found very good quantitative agreement between the data and a fully quantum mechanical bonding model for ice Ih, and substantial disagreement with a purely electrostatic classical bonding model (Figure 2).
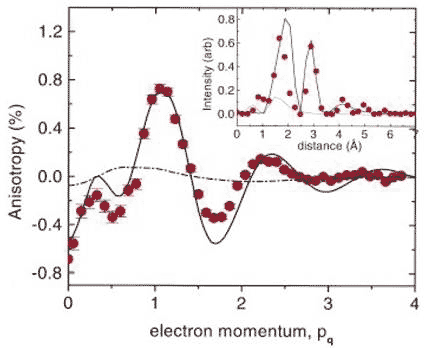
Figure 2 Anisotropy in the Compton profile of ice Ih; inset shows the Fourier transform that identifies the spatial periods of the variation (see text for more details)
In Figure 2, the continuous line is the calculated profile based on a fully quantum mechanical model, which shows good agreement with experimental data (red dots). As in any quantum calculation, covalence implies wave function phase coherence between neighbouring molecules, which leads to the interference fringes represented by the continuous squiggly line. The broken line is the calculated profile based on the classical electrostatic model, which shows little anisotropy and does not agree with the data at all.
If the electrons of the hydrogen bonds are not in a configuration predicted by classical electrostatic model, then the protons too, must be quantum mechanical. In other words, a proton could be in two places (delocalized) along the O-H···O axis linking up the covalent bond of the donor water molecule and the hydrogen bond to the acceptor molecule.
Researchers at the FOM Institute for Atomic and Molecular Physics in the Netherlands used ultrafast femto-second (10-15 s) pulses of infrared light to excite and probe the O-H covalent bond vibration in liquid water, thereby to capture the delocalization of the proton between the oxygen atoms of the two neighbouring water molecules [9]. They found that the energy required for this delocalization is surprisingly low, corresponding to less than 20 % of the dissociation energy of the O-H bond of the water molecule in the gas phase.
This is due to the anharmonic interaction – one in which restoring force is not proportional to displacement – between the O-H stretch vibration of the covalent bond and the hydrogen bond. Using quantum mechanical calculation of the vibrational wave functions, the researchers were able to reproduce the experimental absorption spectrum. A potential diagram along the O-H···O axis based on the model is presented in Figure 3.
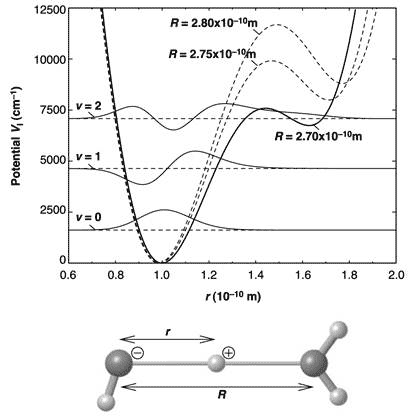
Figure 3 Potential diagram along the O-H···O axis, with the three lowest-energy (v = 0, 1, 2_ vibrational wave functions in the potential for R (O-O distance) = 2.7 Å
The excited proton can be found simultaneously at a distance of the equilibrium O-H bond from the left oxygen in O-H···O, and at the same distance from the right oxygen (in the two potential wells); and at a much decreased energy for the excited (v=2) state than if the hydrogen bond did not exist (as in the gas phase). Hence it also increases the probability of proton transfer. The energy of excitation to the v=2 delocalized state of 6 500 cm-1 (0.82 eV) is less than 20 % of the O-H bond energy of 38 750 cm-1 (4.8 eV).
Recently, researchers led by physicist George Reiter at University of Houston, Texas, obtained direct evidence of both quantum coherence and proton superconductivity of water confined in carbon nanotubes and other nanospaces. They used deep neutron inelastic scattering at the ISIS Facility of Rutherford Appleton Laboratory in Oxford, UK, to measure the distribution of momentum of the protons in the water molecules. In this technique, intense beams of neutrons are fired at the water molecules and scattered from the nuclei of hydrogen atoms, i.e., protons, so that both the energy of the neutron and the proton is changed. The momentum of the proton is mainly determined by the wave-function of the proton’s ground-state (least energetic state).
Reiter and colleagues had earlier used the same technique to look at water confined in single walled carbon nanotube (SWNT) of average diameter 14 + 1 Å (1 Å = 10-10m) [10].
They found that the kinetic energy of the protons is 35 meV less than that of protons in ice Ih at the same temperature of 5 K, and the high momentum tail, characteristic of the O-H covalent bond and the stretch mode of water is completely missing. Measurements of the nanotube water at 170 K and 230 K were indistinguishable from 5 K. At 268 K, however, the high momentum tail is restored. The local structure around the proton has changed to one resembling bulk ice. There is a transition between 230 and 268 K to a 3-dimensional coordinated state that resembles ordinary ice in its kinetic energy (44 meV higher than at 5 K) and in the presence of a high momentum tail. This transition occurs well above the value of 200 K predicted by classical molecular dynamic simulations of the structure.
A fit to the data is obtained with a simple model in which the wave function along the bond direction is the sum of two Gaussian curves separated by a distance d of 0.21 Å. The narrowness of the momentum distribution along the bond direction reflects a coherent delocalization between the two positions.
The precise explanation of the results is still uncertain, but they clearly indicate that the quantum state of the protons confined in narrow nanospace at low temperature is qualitatively different from any phase of water seen up to then.
Water is nothing if not flexible, and a dazzling array of crystalline and quasicrystalline structures have been found in SWNTs of different diameters [11] (see also Chapter 19 of Living Rainbow H2O [4]).
In further experiments, Reiter and colleagues discovered that water confined in space dimension of 2 nm or smaller have protons that are coherently delocalized in two momentum states, in ‘double wells’ [12].
Double walled nanotube (DWNT) of internal diameter 16 + 3 Å behaved quite differently from the 14 Å SWNT. The width of the radial momentum distribution narrows for water in the SWNT, but broadens for water in the DWNT compared with bulk water; this remarkable change being due to a difference in diameter of just 2 Å.
The structure of the water at 170 K as determined by classical simulations consists of a cylindrical square ice sheet about 0.3 nm away from the carbon wall, with a single chain of water molecules down the centre of the SWNT, and in the case of the DWNT, a smaller inner cylinder of water down the centre. Thus, the quantum state of protons in confined water is very sensitive to the nature of the confinement and responds to the global configuration of the H-bond network. Further differences are found in the temperature dependence of the proton momentum distributions. While the momentum distribution of the SWNT water is independent of temperature up to 230 K (see above), that of water in the DWNT is strongly temperature dependent (Figure 4).
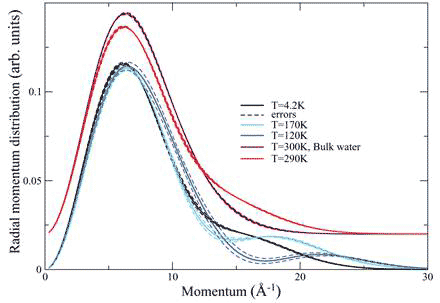
Figure 4 Radial momentum distribution of water in DWNT at different temperatures compared with bulk water at room temperature; the two uppermost curves have been displaced upward by 0.o2 units for clarity
Deep inelastic neutron scattering detects coherent delocalization, as evident in the spreading out of the wave function into a bimodal distribution of a double-well system. A significant number of protons in the DWNT are in that state, and furthermore, the separation of the wells changes with temperature, getting smaller from 4.2K to 120 K and then getting larger again at 170 K.
Confinement effects are also observed in hydrophilic nanospaces, and at room temperature. The interaction of the water with the surface of the nanotube is primarily Coulomb repulsion and van de Waals attraction. There is no chemical bonding between the water and the nanotube walls.
In earlier experiments on xerogel, a glass sponge with Si-OH (slianol) groups lining the surface of pores that can form hydrogen bonds with water [13]. Proton momentum distribution of the water in the 24 Å pores at room temperature could be described as though all the molecules were confined in a double-well potential. For larger pores of 82 Å, the average momentum distribution was closer to that of bulk water, though still quite distinct (Figure 5).
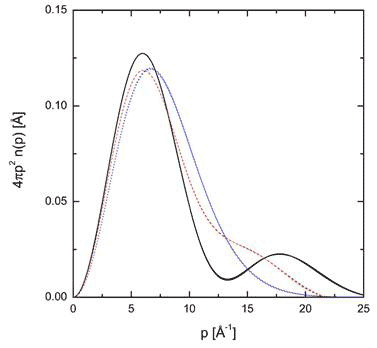
Figure 5 Radial momentum distribution of water in xerogel 24 Å diameter pores (black curve) compared with xerogel r 82 Å diameter pores (red curve) and bulk water (blue curve) all at room temperature
Two systems similar to the xerogel are the perflurosulphonic acid membranes Nafion 1120 and Dow 858. These are ionomers (polymers consisting of repeats of both electrically neutral units and a fraction of ionized units, usually no more than 15 %), with hydrophobic poly(tetrafluoroethylene) (PTFE) backbones and randomly side chains of perfluoroether terminating with sulphonic acids. When hydrated, they exhibit nanophase separation where water ions exist in domains a few nanometres in diameter surrounded by the hydrophobic backbones. The sulphonic acid group (-SO3H) donates its proton to water when there is sufficient water in the pores, making them very good proton conductors.
The momentum distribution at room temperature for the two membranes are dramatic, and corresponds to a kinetic energy difference compared with bulk water of +107 meV/proton for Nafion and +124 meV/proton for Dow 858 [12] (Figure 6).
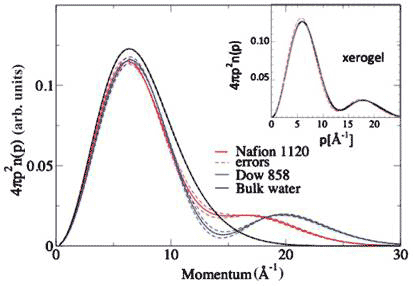
Figure 6 Proton momentum distribution of proton exchange membranes compared with bulk water; inset proton momentum distribution of xerogel (see text)
At a concentration of 14H2O/SO3H for both membranes, Dow858 has a significantly higher conductivity than Nafion, as consistent with the greater proton delocalization, judging by the depth and position of the minimum in the momentum distribution (Figure 5). A proton that delocalizes in two places should have a greater conductivity than one that is localized around a single position; and it does.
The authors concluded [12]: “There is experimental confirmation of correlated proton motion in the vibrational spectrum of ice. We hypothesize that a similar phenomenon is occurring in water, with the correlated motion of the protons producing a response of the electrons that leads to an effective double well for the protons, and that this correlated state, while occurring at higher energies....than the individual molecule state of bulk water, is sufficiently close in energy that it can become the ground state when the hydrogen bond network of bulk water is disrupted by confinement. The changes in the zero-point motion [ground state] of the protons in confined water, as in living cells, for instance, can be expected to play a significant role in the energetics of the cells, where typical distances between components are on the order of 20 A°.”
In other words, confinement itself is sufficient to causes changes in the electronic (bond) structure to excite the ground state of water to coherent delocalization that makes it superconduct protons.
Recent work on the structure of PEMs sheds further light on their proton conducting properties.
The chemical structure of Nafion combines a hydrophobic, Teflon-like backbone with hydrophilic ionic side groups (Figure 7).
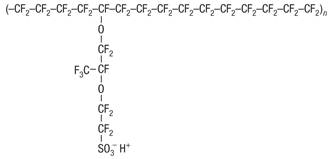
Figure 7 Chemical structure of Nafion
In hydrated Nafion, these components self-organize to produce a clear peak on small-angle X-ray or neutron scattering. Using a recently introduced algorithm, Klaus Schmidt-Rohr and Qiang Chen at Iowa State University, quantitatively simulated the published small-angle scattering data of hydrated Nafion [14]. They showed that the characteristic ionomer peak arises from long parallel, but otherwise randomly packed water channels surrounded by partially hydrophilic side branches, forming inverse-micelle cylinders. (Inverse micelles are formed by detergents with their hydrophilic ends facing inside and hydrophobic ends facing out.) At 20 % by volume of water, the water channels have diameters between 1.8 and 3.5 nm, with an average of 2.4 nm. Nafion crystallites (small crystal structures) constituting ~10 % of the volume, form crosslinks that are crucial for the mechanical properties of Nafion films, and are elongated and parallel to the water channels, with cross-sections of ~5 nm2 (Figure 8). A dozen other models fail to match the experimental scattering data.
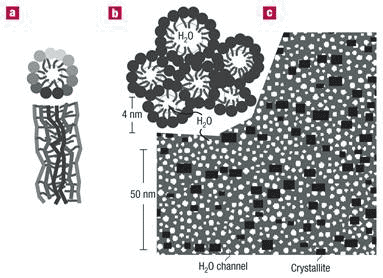
Figure 8 Parallel water channel (inverse-micelle cylinder) model of hydrated Nafion; a, an inverse micelle cylinder in cross section and longitudinal section; b, approximately hexagonal packing of water channels; c, Cross-section through the cylindrical water channels (white) and the Nafion crystallites (black) in the non-crystalline Nafion matrix (grey)
This structure is not unlike that of the cytoplasm, the water being distributed in fractal spaces approximated by a mathematical Sierpinski sponge (see Chapter 18 of [5]).
Although about half the water in Nafion appears to freeze at ~-20 C, conductivity and diffusion in Nafion persist below -2 C down to -50 C, with moderately larger activation energy than at higher temperatures.
Nafion films have a proton conductivity of about 0.1 S/cm (S, Sieman = 1 Ampere/Volt); among the highest in PEMs. (For comparison, the electrical conductivity of copper, one of the highest, is 596 000 S/cm, that of water 0.00055 S/cm, and silicon, a semi-conductor, 0.156 S/cm [15].) But the conductivity of a single high purity Nafion nanofibre made by electrospinning reached 1.5 S/cm, an order of magnitude greater, as Yossef A Elabd at Drexel University Philadelphia in Pennsylvania, USA, and his colleagues showed [15]. They also observed an effect of confinement, where proton conductivity increases sharply with decreasing fibre diameter. An oriented ionic morphology was observed in the nanofibre in contrast to the isotropic morphology in the bulk film.
Electrospinning is one technique that can produce polymer fibres nanometres in diameter by applying a high-voltage electric field to a polymer solution ejecting out of a metal syringe needle. The high-purity Nafion nanofibres made by electrospinning had only 0.1 wt % of carrier polymer, polyethylene oxide (MW 8 000); and proton conductivity as high as 1.5 S/cm was found at a fibre diameter of 400 nm. This is due to alignment of interconnected ionic aggregates along the fibre axis direction as evidenced by X-ray scattering. Also, an order of magnitude increase in humidity sensitivity was observed in Nafion nanofibres compared with the bulk film.
As shown in Figure 9, the conductivity of the Nafion fibres increases exponentially as fibre diameter decreases (left graph). The proton conductivities of the fibres with diameters >2 μm was similar to the bulk Nafion film (∼0.1 S/cm). However, when the fibre diameter was <1 μm, proton conductivity increased sharply with decreasing fibre diameter and reached a value as high as 1.5 S/cm for the 400 nm diameter nanofibre; at least an order of magnitude higher than the bulk Nafion film. Conductivity of the fibre also increases a hundred fold as relative humidity rises from 50 to 90 % (Fig. 8 right graph); in comparison, conductivity of the bulk film has increased only 10 fold.
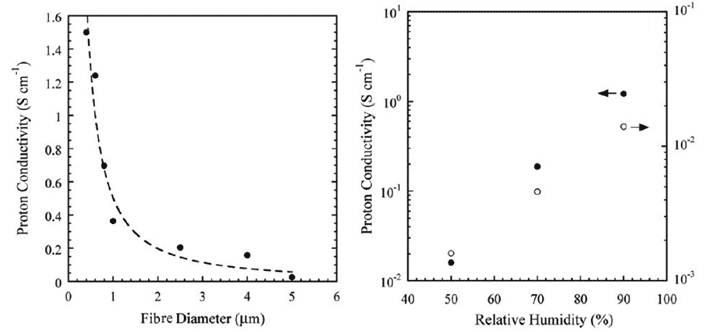
Figure 9 Conductivity of Nafion nanofibre as a function of diameter (left) and relative humidity (right)
The inverse micelle model of Nafion is especially relevant to the living cell, where interstices between fibres of the cytoskeleton and cytoplasmic membranes effectively form inverse micelle nanospaces and channels that are now known to drastically alter enzyme/substrate relationships and enzyme activity compared to bulk phase thermodynamic models that still dominate conventional cell biology (see Chapter 18 of Living Rainbow H2O [4]). The model may be even more relevant to the extracellular milieu of multicellular animals, which is traversed by aligned collagen fibres consisting of fibrils interwoven with nanotubes of water [16] (see Chapter 20 of Living Rainbow H2O [4]). These water channels aligned with collagen fibres are most likely the anatomical correlates of the acupuncture meridians of traditional Chinese medicine, as I and David Knight first suggested in 1998 [17] and the hypothesis is still very much alive and untested ([18] Acupuncture, Coherent Energy and Liquid Crystalline Meridians, ISIS Lecture). It would be a simple matter to measure the proton conductivity of single collagen fibrils for a start.
Article first published 14/07/12
Comments are now closed for this article
There are 4 comments on this article.
Robert Davidson, MD PhD Comment left 17th July 2012 01:01:50
Great post, Maewan! It's clear now that the pure science underlying the existence of this new 4th phase of water or "neo" water is growing by leaps and bounds. Naysayers should remember the moment! Acupuncture, grounding, and homeopathy are alive and well. Complementary, alternative, and integrative medicine are sustainable. The allopathic model of healthcare is broken. Your post explains why.
Nigel Dyer Comment left 18th July 2012 01:01:49
I agree that there is extensive evidence for proton delocalisation associated with the H bond. However, I am less convinved that this leads directly to the increased conductivity in water that is seen. Instead my hunch is that the delocalisation allows the formation of a more extended structure of water with delocalised electrons, and it is this that causes the increased conductivity
Jayney Goddard Comment left 18th July 2012 01:01:52
Agree Robert Davidson, great post Maewan! And, many thanks for taking the time to do this, it is greatly appreciated. More please!
Todd Millions Comment left 18th July 2012 18:06:48
Facinating-I still recall gratefully your exellent exposition of the Chapman water model.
Would any work have being done on collegen based super conducting-wires?I ask because while it might not be room tempture-if continuous enough for wires,a higher than liquid nitrogen performance would be very useful(non-"don't slow down the scans,and vent the helium"MRI comes to mind.).The brittleness of metallic or ceramic based versions,shouldn't be a limit with collagen,as any one who has hand sawed frozen joints of meat could tell you.Thanx